|
Pharmacogn Rev. 2022;16(31):45-61 A multifaceted peer reviewed journal in the field of Pharmacognosy and Natural Products www.phcogrev.com | www.phcog.net |
Review Article |
Spondias mombin L.: An Updated Monograph
Ana Clara Brito Maria1, Thaíse Reis Simões1, Aline de Souza Ramos1, Maíra Martins Haddad de Almeida1, Maria Athana Mpalantinos da Silva1, Jefferson Diocesano da Cruz1, José Luiz Pinto Ferreira2, Jefferson Rocha de Andrade Silva3, Ana Claudia Fernandes Amaral1,*
Ana Clara Brito Maria1, Thaíse Reis Simões1, Aline de Souza Ramos1, Maíra Martins Haddad de Almeida1, Maria Athana Mpalantinos da Silva1, Jefferson Diocesano da Cruz1, José Luiz Pinto Ferreira2, Jefferson Rocha de Andrade Silva3, Ana Claudia Fernandes Amaral1,*
1Laboratory of Medicinal Plants and Derivatives, Department of Natural Products, Farmanguinhos, Fiocruz, Rio de Janeiro, BRAZIL.
2Faculty of Pharmacy, Federal Fluminense University, Niterói, BRAZIL.
3Chromatography Laboratory, Department of Chemistry, Federal University of Amazonas, Manaus, BRAZIL.
1Correspondence
Dr. Aline de S Ramos,
Laboratory of Medicinal Plants and Derivatives, Department of Natural Products, Farmanguinhos, Fiocruz, Rio de Janeiro, BRAZIL.
E-mail: [email protected]
2Correspondence
Dr. Ana Claudia F Amaral,
Laboratory of Medicinal Plants and Derivatives, Department of Natural Products, Farmanguinhos, Fiocruz, Rio de Janeiro, BRAZIL.
E-mail: [email protected]
History
• Submission Date: 03-09-2021;
• Review completed: 05-10-2021;
• Accepted Date: 17-11-2021.
DOI : 10.5530/phrev.2022.16.8
Article Available online
http://www.phcogrev.com/v16/i31
Copyright
© 2022 Phcog.Net. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International license.

ABSTRACT
Spondias mombin Linn (Anacardiaceae) is a plant species found from southeastern Mexico to Peru and in the northern of Brazil. Its fruits are known as “cajá miúdo” or “cajá pequeno” in Brazil; in Central America, as “jobo”; as “hogplum” or “yellow mombin” in North America; and as “ciruella amarilla” in Mexico and Ecuador, among others. It is used by the local population to treat diseases due to its many biological properties, such as antiviral, anti-inflammatory, antioxidant, and antibacterial properties. This study was carried out using databases, indexed articles, theses, dissertations, and books. These surveys indicate important microscopic and macroscopic patterns of the species. Some studies also confirmed the popular use of S. mombin, showing in vitro and in vivo antioxidant properties and some activities, such as mosquito adulticidal, anti-inflammatory, antiviral, antibacterial, and anthelminthic activities. These properties are attributed to the abundant presence of phenolic compounds. Saponins, alkaloids, phenolic acids, tannins, and flavonoids are also found in its extracts. The results obtained in this study show several pharmacological applications that can lead to the development of new products and drugs based on the properties of S. mombin, which have already been reflected in field patents.
Key words: Anacardiaceae, Botanical description, Chemical composition, Pharmacology, Phytochemical analysis.
Cite this article: Maria , A. C. Brito, Simões, T. Reis, Ramos, A. de Souza, de Almeida, M. Martins Haddad, da Silva, M. Athana Mpalantinos, da Cruz, et al. Spondias mombin L.: An Updated Monograph, Pharmacog Rev. 2022;16(31):45-61.
INTRODUCTION
Spondias mombin Linnaeus (Anacardiaceae) is a plant species found from southeastern Mexico to Peru and in the northern and northeastern regions of Brazil. S. mombin is commonly known as “ciruella amarilla” in Mexico and Ecuador; “cajá” in Brazil; and “yellow mombin” in North America, among others.[1,2] Some of the medical uses include the leaves in the treatment of wounds and inflammations; the infusion preparation of its flowers against stomachache, constipation, and bilious fevers; the decoction of the roots as antidiarrheal; and the decoction of its barks to treat emesis, diarrhea, and hemorrhoids.[3-5] Many studies have shown important properties in S. mombin, such as antiviral, anti-inflammatory, antioxidant, and antibacterial properties, mainly attributed to flavonoids, saponins, and phenolic acids isolated from leaves.[6-9]
In 2004, our research group developed a monograph as a narrative review of the chemical properties of S. mombin that was published as a book chapter.[10] However, there are many other properties described in the literature, including in vivo and in vitro studies, that were not covered in our previous chapter or another review article. Thus, this study aimed to gather new information about the properties of S. mombin and the progress of new products containing this plant species, contributing to the scientific community.
METHODS
Systematic studies were conducted using Science Direct, Scholar Google, and PubMed as databases. The keywords used to find the articles were “Spondias mombin” combined with “activities”, “biological activities”, “formulation”, “nanoparticle”, “nanotechnology”, “popular use”, “folk medicine”, “chemical profile”, “constituents”, “physicochemical analysis”, “botanical”, “distribution”, “uses”, “popular names”, and “characterization”. The year of publication was not used as a criterion, and the language was limited to Portuguese and English.
In the search for patents, a data survey was carried out in April 2021 in the following patent search databases using “Spondias mombin” as the keyword: European Patent Office (EPO), United States Patent and Trademark Office (USPTO), World Intellectual Property Organization (WIPO) and Instituto Nacional de Propriedade Industrial (INPI).
BOTANICAL ORIGIN
Spondias mombin Linnaeus belongs to the Anacardiaceae family, which includes 73 genera and approximately 850 species.[11] Some synonyms are S. aurantiaca Schum and Thonn, S. axillaris Roxb., S. dubia A. Rich., S. graveolens Macfad., S. lutea var. glabra Engl., S. lutea var. maxima Engl., S. lutea var. pseudomyrobalanus (Tussac) Marchand, S. nigrescens Pittier, S. pseudomyrobalanus Tussac, S. oghigee G. Don, S. purpurea var. venulosa Engl., S. radlkoferi Donn. Sm., S. venulosa (Engl.) Engl., S. zanzee G. Don.[12]
POPULAR USES
All parts of S. mombin can be used for nonmedicinal or medicinal purposes. Among the nonmedical uses, bark is used in carving Figures and as a dyeing agent, while leaves are used as vegetables. Flowers are used for decoration, and nectar can be used for honeybees. The root is considered a water source, while the stems are used in living farmland and as a shelter by artisans. Wood is used in carpentry to make different objects, such as pencils and match sticks. In Brazil, it is used as a bottle stopper and in the manufacture of sealing wax. In Costa Rica it is also used as fuel. Wood ash was used as an indigo dye, and gum was used as glue. The fruits are used to alleviate thirst; to prepare ice cream, cool beverages, and jelly in Costa Rica and Brazil; to produce wine in Amazon - known as “Vinho de Taperiba” - and a cider-like drink in Guatemala.[3]
The medicinal uses described in the literature are summarized in Table 1.
Table 1: Medicinal uses of Spondias mombin L.
| Medicinal uses of S. mombin L. | |||
|---|---|---|---|
| Used part | Mode of use | Medicinal use | Ref. |
| Sprouts | Decoction | Treatment of Erysipelases Feet’s Swelling | [60] |
| Sprouts | Juice | Cataract treatment | [61] |
| Stem (barks) | Decoction | As emetic, astringent, tonic, antidiarrheal, stimulant, antispasmodic, dysentery, gonorrhea, antitussive, colic, vaginal inflammation, hemorrhoids, uterus tonifying, antiviral, antimalarial, muscle analgesic, relieve tiredness, contraceptive, to treat metrorrhagia, poly menorrhea, and to expel calcifications from the bladder. | [3,62-69] |
| Stem (barks) | Infusion | Anti-inflammatory, anti-diarrheal, healing, anti-colic, excessive menstruation bleeding, intestinal ulcers, vaginal and stomach infections and dermatitis. | [67,70-72] |
| Stem (barks) | Powder | To heal wounds | [3] |
| Stem | Dust | To reduce local bleeding. | [70] |
| Stem | Resin | Healing and to treat minor burnings | [62,70] |
| Root | Decoction | Purgative, antidiarrheal and used in women who have just given birth. | [5,73] |
| Flower | Decoction | Treatment of laryngitis, conjunctivitis, heart palpitations and as a tonic. | [60,74] |
| Flower | Infusion | Against stomach ache, constipation, bilious fevers, cystitis, eye and laryngeal diseases | [4] |
| Leaves | Decoction | As anti-inflammatory in mouth and throat, treatment of cold sores or genital, prostatitis, breast enlargement, antipyretic | [4,19,68-70,75-77] |
| Leaves | Infusion | Against stomach ache and infections, fevers, cystitis, urethritis, eye and laryngeal diseases, vaginal infection, dermatitis, diarrhea, gout, herpetic angina and constipation. | [4,19,68,70,76,78]] |
| Leaves | Extract | It has antimicrobial activity; smooth muscle relaxant, uterine stimulant, antifertilizing and antiviral activity. In cataract treatme | [76,79] |
| Fruit | In natura | Antispasmodic, against erysipelas, anemia. Against cardiac hypertrophy, angina, uterus and vagina ulcers. Local anti-inflammatory in knees. In large quantities it acts as an emetic. | [4,76,80] |
| Fruit | Macerated | Against cystitis, urethritis and emetic. | [62,74] |
| Fruit | Dust | Acts as an emetic. | [76] |
| Fruit | Juice | Anti-pyretic, anti-inflammatory, healing and anti-diarrheal | [71,75] |
| Seed | Decoction | Bladder infections; ulcers and skin disorders. Against the white flow in women. | [75,81] |
| Gum | _ | As expectorant and to expel tapeworm | [1,35,82-85] |
| Seed | Fumigation | Against ulcers and skin disorders. | [86] |
POPULAR NAMES
The fruits of S. mombin are known as “cajá miúdo” or “cajá pequeno” in Southeast and Southern Regions of Brazil and as “taperebá” and “acajá” in the Amazon region. The names “taperebá” and “acajá” are of indigenous origin (Tupi): the first word means “tapir fruit” (from “tapir”, tapir and “iba”, fruit) and the second “stone fruit” (from “acâ”, stone, and “ya”, fruit).[2] Reports in the literature indicate that S. mombin was cultivated by indigenous people in the 16th and 17th centuries.[13,14] Other common names among regions are “prunier mombin” in French Guiana, “ciriguela del monte” and “jacote” in Guatemala, “azucaró” and “cedrinho” in Bolívia, “ciruella amarilla” in Mexico and Ecuador. The fruit is also known as “jobo” in Central America and Venezuela, as “hogplum” or “yellow mombin” in North America, as “ambaló” in Goa, and as “munguengue” in Angola.[15] Other vernacular names are “macaprein”, “hoba” and “yellow plum” in Netherlands Antilles; “jobillo” and “jobo vano”, “jobo de perro” in Puerto Rico; “ciruela”, “joboban”, “jobo de poerco” in Dominican Republic; “mope”, “moppe”, “hooboo” in Suriname; “risco”, “orocoro-cillo”, “hobo”, “jobo”, “jobo blanco”, “jobo colorado”, “jobo arisco”, “jobo del amazonas”, “jobo de castilla” in Colombia and “job female” in Cuba.[16]
GEOGRAPHICAL DISTRIBUTION
S. mombin is native to moist lowland forests from southern Mexico to Peru and Brazil and is also found in many of the West Indies. It is widely cultivated and naturalized in tropical Africa.[1] The species is found in Chile, Argentina, Colombia, Bolivia, Panama, Nicaragua, Suriname, Ecuador, French Guiana, Puerto Rico, Guyana, Paraguay, Uruguay, Honduras, Guatemala, and Venezuela.[17] In Brazil, its occurrence is confirmed in almost all regions, mainly in the North and Northeast, and except in the South.[15,18]
BOTANICAL DESCRIPTION
Macroscopic Description
S. mombin is a perennial fructiferous tree that can reach 15 - 22 m. It is erect, the cylindrical trunk measures approximately 2 m in circumference and is covered by a very thick, rough, grayish, or whiteish bark.[19] The branches are glabrous, from 4 m above ground height. The wide canopy is spread, sometimes densely closed, varying from 8 to 24 m high.[20] Its trunk can often produce a brown resinous substance.[3,21]
The flowers can be characterized by four types, including hermaphrodites, males, and two types of females. They are pedunculated, apopetal, actinomorphic, dichlamid, measuring approximately 0.5 cm in diameter, with two bracteoles. There are 5 cyclic sepals with tiny green lobes and 5 petals, unduplicated light yellow, measuring 0.3 cm long and free, valvar; there are ten stamens with extruded anthers, with subglobose, basifixed, rhymed and fimbriated stigma.[20,22]
The fruits are small with an elliptical form of 3 - 4 cm,[23] and are considered drupaceous berries or pseudodrupes. There is great variability in size, color, aroma, and flavor.[24] There is a tapered and round end in S. mombin’s endocarps, and they can be elliptical, obovoid, ovoid, and globose. In the cross-section, it is possible to observe spongy fibers and seeds through a radial structure of woody consistency and five radially arranged locules. The seeds are elongated, elliptical and of different lengths, with a small integument.[25]
The leaves are pointed, alternating, with 5 - 11 pairs of leaflets, with a petiole measuring 5 cm in length; opposite or alternate leaflets; midrib prominula on the upper surface, glabrous, with much pelage on the back; campitodromous cladodromous nerve, with 16 - 18 pairs of secondary ribs; the rachis is 20 – 30 cm long, and there are no stipulations. The rachis and petioles are often reddish in color; the leaves are in helical distribution and clustered toward the tips of the branches, also characterized by the smell of green mango when pressed.[20,24]
Microscopic Description
The primary stem, when transversely sectioned, presents a unistratified epidermis of quadrangular contour cells with small, conical, and unicellular tector trichomes, subepidermal collenchyma, cortical parenchyma with rounded outline, cells and scleral cells isolated or in groups; scleral ring is composed of thick-walled stone cells; there are also collateral vascular bundles with the xylem arranged in radial rows containing secretory structures. Calcium oxalate crystals are present in the collenchyma, cortical parenchyma, and phloem region.[26]
The epidermis of the leaves shows square cells with straight walls and thick cuticles, rare conical tector trichomes, and short, unicellular and anomocytic stomata in the abaxial epidermis. The asymmetric heterogeneous mesophyll shows a single layer of palisade cells and spongy parenchyma. Tector trichomes appear in the midrib. Stone cells compose the subepidermal collenchyma tissue and the fundamental parenchyma. Calcium oxalate druses are present in the subepidermal collenchyma and fundamental and spongy parenchyma tissues. The xylem vessels are arranged radially, surrounded by phloem and by a fibrous pericycle on the outside. From the outside to the inside, the petiole contains pubescent epidermis with unicellular tector trichomes and filling tissue with rounded cells containing calcium oxalate druses, vascular bundles, and medullary parenchyma.[27]
A study on the microscopy of S. mombin barks will also be valuable to enrich further studies, as there are some descriptions of its popularity and some studies have demonstrated its secondary metabolites and other properties related to bark.
CHEMICAL CONSTITUENTS
Before performing the biological assays, it is important to highlight the extraction method, isolation, and detection/identification of the chemical constituents of the extracts. The summary of this section, as well as their respective results, is shown in Table 2.
Table 2: Summary of phytochemical assays performed with S. mombin and respective results obtained on phytochemical assays.
| Summary of performed phytochemical assays and their respective chemical constituents and/or the detected classes of metabolites | |||||
|---|---|---|---|---|---|
| Part used | Extraction and fractionation method | Analytical and identification methods | Extraction and fractionation details | Chemical constituents or chemical classes | Ref. |
| Fresh leaves | β-caryophyllene (27.9%); γ-cadinene (12.3%); β-cadinene (7.8%); caryophyllene oxide (6.9%); 5-isocedranol (6.4%); α-gurjunene (6.4%); neral (6.2%) | ||||
| Hydrodistillation | GC-MS (HP-5MS) | [30] | |||
| Air-dried leaves | β-caryophyllene (30.9%); γ-cadinene (9.7%); β-cadinene (6.6%); caryophyllene oxide (6.2%); 5-isocedranol (9.5%); α-gurjunene (7.3%); neral (9.4%) | ||||
| Leaves and Stems (together) | 1)Maceration; 1.1) Partition; 1.2) Column cromatography (n-BuOH/MeCOEt extract); 1.3) Column cromatography; 1.4) Countercurrent cromatography; 2) Column cromatography; 2.1) Prep. TLC; 3) Prep. TLC; 3.1) Column cromatography; 4) Column cromatography; 4.1) Vacuum liquidum cromatography; 4.2) Prep. TLC | Nuclear Magnetic Resonance (NMR) 1 H and 13CNMR | 1) Ethanol 80%; 1.1) CCl4, Et2O, n-BuOH/MeCOEt (1:1); 1.2) Sephadex LH-20, H2O, MeOH, MeOH/Me2CO (1:1); 1.3) silica gel, MeOH, MeOH/Me2CO (1:1); 1.4) CHCl3/MeOH/iso-PrOH/H2O (8:12:1:9); 2) silica gel, CHCl3/MeOH/H2O; 2.1) silica gel, CHCl2/Me2CO/HCOOH/H2O (10:7:1:1); 3) silica gel, CHCl3/Me2CO/HCOOH (75:17:8); 3.1) Sephadex LH-20, 95% MeOH; 4) silica gel, CHCl3/MeOH/H2O 4.1) silica gel, CHCl2/MeOH/HCOOH; 4.2) Sephadex LH-20, 95% MeOH | Chlorogenic acid (30 mg); chlorogenic acid n-butyl ester (50 mg); caffeoyl ester (10 mg) | [87] |
| Leaves and Stems (together) | 1) Maceration; 1.1) Partition; 1.2) Vacuum liquidum cromatography (CCl4 extract); 1.3) Column cromatography (CHCl3 fraction); 1.4) Column cromatography; 1.5) Column cromatography; 2) Partition (residue of step 1); 2.1) Precipitation (petroleum ether fraction); 2.2) Column cromatography | TLC (sulfuric anisaldehyde); 1H and 13C-NMR. | 1) Ethanol 80%; 1.1) CCl4, Et2O, n-BuOH/MeCOEt (1:1); 1.2) silica gel, petroleum ether, CHCl3, Me2CO, MeOH, 90% MeOH; 1.3) silica gel, hexane:CHCl3 (8:2), CHCl3, CHCl3-Me2CO (1:1); 1.4) silica gel, petroleum ether:EtOAc:HCOOH from 95:4:0.5 to 60:40:0.5; 1.5) Sephadex LH-20, CHCl3:MeOH (1:1); 2) petroleum ether, 20% MeOH; 2.1) Pb(OH)2; 2.2) Lobar B column, Lichroprep RP-18, MeOH, 5% HCOOH from 85:15 to 99:1; silica gel, hexane:EtOAc:HCOOH (85:12:2) | 6-(8’Z, 11’Z, 14’Z-heptadecatrienyl)-salicylic acid; 6-(8’Z, 11’Z-heptadecadienyl)-salicylic acid; 6-(10’Z-heptadecenyl)-salicylic acid; 6-(12’Z-nonadecenyl)-salicylic acid; 6-(15’Z-heneicosenyl)-salicylic acid | [84] |
| Seeds (pulverized) | 1) Maceration | GC-MS (HP5-MS column) | Methanol:water (70:30) | Dodecanoic acid (22.5%); tetradecanoic acid (17.9%); n-hexadecanoic acid (15.3%); capsaicin (12.1 %) | [29] |
| Leaves (dry and powdered) | 1) Cold maceration; 1.1) Adsorption; 1.2) Column cromatography (Dichloromethane fraction) | HPLC (C18, water, methanol); HPLC/ESI-MS (C18, 0.1% formic acid in water, methanol) | 1)Methanol; 1.1) silica gel, hexane, dichloromethane, ethyl acetate, acetone, methanol 1.2) silica gel: nonpolar to polar solvents | Ellagic acid; 1-O-Galloyl-6-O-luteoyl-α-D-glucose | [40] |
| Leaves | 1) Maceration; 1.1) Column Cromatography | HPLC (100RP18, phosphoric acid solution,acetonitrile); 1H and 13C-NMR | 1) Methanol:water (80:20); 1.1) silica gel; hexane, chloroform, and ethyl acetate | Quercetin (2.36 mg/g); ellagic acid (41.56 mg/g) | [6] |
| Leaves (dried and powdered) | 1) Maceration; 1.1) Column Cromatography | TLC (ferric chloride, NP reagent, sulfuric vanillin); LC-DAD-MS/MS (C18, water, acetonitrile, formic acid) | 1) Ethanol:water (35:65); 1.1) Sephadex LH-20, water:methanol (70:30) to methanol (100 %) and methanol:acetone (50:50) | Quercetin (2.36 mg/g); ellagic acid (41.56 mg/g) | [32] |
| Leaves (dried and powdered) | 1) Maceration 2) Turbolysis | HPLC-DAD (C18, acetonitrile, phosphoric acid 0.2%, triethylamine 0.2%) | Ethanol/water 70:30 | Chlorogenic acid (0.033-0.083%); geraniin (0.21-0.74%) | [33] |
| Leaves (dried and triturated) | 1) Maceration; 1.1) Partition | HPLC-DAD (C-18, acetonitrile, acetic acid) | 1) Ethanol/water (70/30); 1.1) Hexane, dichloromethane, ethyl acetate, butanol | Chlorogenic acid (0.033-0.083%); geraniin (0.21-0.74%) | [7] |
| Leaves (air-dried) | 1) Cold maceration | TLC (KOH, NEU, Dragendorff, vanillin/sulfuric acid reagents); HPLC (C18, water, methanol) | Hexane, ethyl acetate, ethanol | Flavonoids, cinnamic derivatives, triterpenoids, steroids, mono- and sesquiterpenes, alkaloids, proanthocyanidins and leucoanthocyanidins. Gallic acid (101.52 μg/mL); ellagic acid (68.74 μg/mL) | [41] |
| Leaves (powdered) | 1) Cold maceration; 2) Successive solvent extraction | According to Harbourne[88] | 1) Methanol; 2) n-hexane, ethyl acetate, methanol | Tannins, saponins, flavonoids, proteins, glycosides, resins, triterpenes and steroids | [44] |
| Leaves | 1) Maceration; 1.1) Solvent Extraction | HPLC (C18, acetonitrile, acetic acid 1%); GC-MS (ZB-5 column) | 1) Ethanol/water (7/3); 1.1) Hexane | Ellagic acid, isoquercitrin and sitosterol | [47] |
| Leaves (air-dried pulverized) | 1) Soxhlet extraction; 2) Infusion | According to Trease, Wall[89-91] | 1) Ethanol/methanol (1:1); methanol/water (1:1); 2) boiled distilled water | Tannins, anthraquinones, flavonoids, glycosides, saponins and phenolic compounds | [49] |
| Leaves | 1) Solvent extraction | HPLC-DAD (ODS, 0.1% formic acid in water, acetonitrile) | Hydroethanol 70% | Quercetin (15.6 µg/mL); isoquercetin; resveratrol (1.146 µg/mL); kaempferol (3.01 µg/mL) | [50] |
| Barks (Dried and crushed) | 1) Solvent extraction | Precipitation/complexation methods | Ethanol | Saponins; phenols; tannins. | [51] |
| Seeds (oven-dried and pulverized) | 1) Cold maceration 2) Cold maceration | According to Sofowora[92] | 1) Ethanol; 2) methanol | Polyphenols, anthraquinones, reducing sugar, alkaloids, polyphenols, saponins, tannins, flavonoids, glycosides and phlobatannins | [39] |
Table 2 shows the chemical composition studies focused on the extracts obtained from leaves of S. mombin prepared by maceration using a hydroethanolic solvent.
Regarding the classes and compounds most frequently identified and/or isolated, there are several phenolic compounds, especially ellagic acid, chlorogenic acid, and quercetin derivatives (Figure 1).
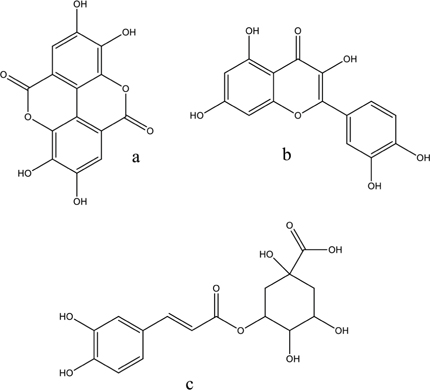
Figure 1: Phenolic compounds found in S. mombin leaves; a: ellagic acid; b: quercetin; c: chlorogenic acid.
It is important to emphasize that these were the classes and compounds most found due to the choice of the extraction method (maceration) and solvents (polar). These metabolites can be altered by changing these factors, as observed by the presence of terpenes through the hydrodistillation process.
PHYSICAL AND CHEMICAL ANALYSIS
In physical characterization, Mattieto et al. (2010),[28] measured 100 fruits of S. mombin, obtaining an average length of 2.93 ± 0.60 cm, width of 2.18 ± 0.27 cm, weight of 7.19 ± 3.20 g, and density of 0.94 ± 0.38 g/cm³. The mass yields represented 24.2 % pulp, 13.8 % bark, 51.8 % seeds, and 10.2 % losses.
The results of the physical-chemical characterization of the pulps were 2.53 ± 0.01 (pH), 1.86 ± 0.01 % citric acid (total titratable acidity), 10.09 ± 0.00 ºBrix (soluble solids), 5.42 ± 0.01 (ratio), 89.42 ± 0.18 % (moisture), 0.82 ± 0.01 % (protein), 0.26 ± 0.09 % (total lipids), 0.58 ± 0.02 % (ash), 1.18 ± 0.10 % (dietary fiber), 0.43 ± 0.12 % (insoluble fiber), 0.75 ± 0.12 % (soluble fiber), and 4.54 ± 0.25 g. 100 g-1 (total sugars), 4.25 ± 0.34 g. 100 g-1 (reducing sugars), 0.29 ± 0.27 g. 100 g-1 (nonreducing sugars), 28.30 ± 0.18 µg. g-1 (total carotenoids), 299.81 ± 0.48 mg. 100 g-1 (tannins), and 23.72 ± 0.08 mg ascorbic acid. 100 g-1 (vitamin C).[28]
In addition, the spectrophotometer results were 61.02 brightness, 14.73 chromaticity +a (red), and 41.50 chromaticity +b (yellow), which showed the predominance of the yellow color in the fruit.[28]
The total phenolic content of the methanol extract of S. mombin fruit (MESSM) was determined using Folin-Ciocalteu phenol reagent and resulted in 239.50 ± 7.9 mg gallic acid equivalents/g. The total flavonoid content was determined by the aluminum chloride chelation method and indicated 105.3 ± 3.6 mg rutin equivalents/g. For antioxidant predictions, the 50% inhibitory concentration (IC50) was calculated through 2,2-diphenyl-1-picrylhydrazyl (DPPH), hydrogen peroxide, and nitric oxide assays, indicating 58.64 ± 1.49, 44.03 ± 5.57 and 494.55 ± 12.68 µg/mL, respectively, and the control (ascorbic acid) showed values of 4.31 ± 0.26, 10.63 ± 0.31 and 48.74 ± 1.46 µg/mL, respectively. According to the authors, it appeared to be the first experiment reporting the probable antioxidant properties with these tests, which shows that there is much to be studied about S. mombin.[29]
The hydroxyl radical scavenging and the reducing power assay of the essential oil (EO) of S. mombin leaves obtained by Oladimeji et al. (2016)[30] were analyzed by two methods: one using ferrous sulfate, H2O2, and salicylic acid and the other using potassium hexacyanoferrate, trichloroacetic acid, and ferric chloride (FeCl3). The EO produced 83 % (fresh leaves) and 99.8 % (dried leaves) scavenging effects (hydroxyl radicals) at a concentration of 1 mg/L, similar to Vitamin C, which exhibited approximately 90 %. These tests showed that EO is effective in halting the oxidation of cellular macromolecules and that its volatile compounds depend totally on scavenging hydroxyl radicals.
Two studies described in the section “Anti-inflammatory activity” have verified the antioxidant properties. Gomes et al. (2020)[8] prepared a hydroalcoholic extract (HE) from S. mombin leaves and measured the total glutathione levels (GSH) in the buccal mucosa of hamsters by adding ethylenediamine tetraacetic acid (EDTA) solution and after mixing the supernatant with Tris buffer (pH 8.9) and 5,5′-dithiobis-(2-nitrobenzoic acid in each sample (4 per group). The values were determined through absorbance measurement at 420 nm. The results demonstrated the ability of S. mombin to prevent the reduction of GSH levels in the buccal mucosa. It is important to highlight the presence of two major phenolic compounds in the extract with potent antioxidant properties: chlorogenic acid (19.4 mg/g) and ellagic acid (12 mg/g).
The study performed by Cabral et al. (2016),[7] showed that HE, the butanol extract, and chlorogenic acid from the leaves were able to sequester 74.53, 73.71, and 91.47 % of free radicals, respectively, according to the DPPH assay. In addition, all these samples could sequester more than 100 % when tested for hydroxyl radical scavenging activity, similar to gallic acid at 15 mg/mL (control). This result also contributes to the suggestion that the antioxidant properties are related to these phenolic acids.
Other researchers who verified the antioxidant properties were Cristofoli et al. (2019).[9] They prepared some extracts from S. mombin leaves: one of them was carried out through a Soxhlet apparatus (Sox) using 150 mL of hexane, ethyl acetate, ethanol, water, or a mixture of ethanol:water (1:1 v/v). Another extraction was conducted through ultrasound-assisted extraction (UAE) using the same solvents in the same proportion for 8 min with a 50 % sonication amplitude (250 W). Then, supercritical fluid extraction (SFE) was performed with 15 g of the sample and CO2. After the extractions, the total phenolic content (TPC) was determined by the Folin-Ciocalteu method, and the antioxidant properties were verified through the free radical scavenging activity by DPPH and the ABTS+ [2,2-azino-bis-(3-ethyl benzothiazoline-6-sulfonic acid)] method.
The best yields were obtained using a Soxhlet apparatus with ethanol (22.5 ± 0.5 %), ethanol + water (23.5 ± 4.4 %), and water (21.6 ± 4.7 %) as solvents and by UAE using ethanol + water (30.5 ± 0.7 %) and water (27.5 ± 1.7 %) as solvents. Considering the highest TPC values, the extracts obtained through the Soxhlet apparatus (EtOH, EtOH + water, and water), SFE (residue), and UAE (EtOH) resulted in a range between 293.0 ± 6.9 and 410.1 ± 33.1 mg GAE/g extract, which were also considered good.[9] This study demonstrated that different techniques and solvents used in the extractions are extremely important to achieve the desired results.
BIOLOGICAL ACTIVITIES
Antiviral Activity
Silva et al. (2011)[6] conducted a study in which they evaluated the antiviral activity of S. mombin against the type-2 dengue virus (DENV-2) replicated in C6/36 cells after phytochemical analysis. Cell viability and antiviral activity were evaluated by the MTT method.
No cytotoxic effect was observed through the MTT method at concentrations up to 1000 µg/mL. Additionally, the crude extract and the isolated substances were tested against DENV-2. At a concentration of 500 µg/mL, ellagic acid and quercetin were able to inhibit viral DENV-2 replication by 25.02 and 50 % in C6/36 cells, while the crude extract was less active, with 3.31 % inhibition.[6] Thus, ellagic acid had better results than quercetin and the crude extract, and it also suggests the potential use of both phenolic compounds, especially quercetin, as an anti-DENV agent.
In contrast to this result, Lima (2015)[31] also studied an extract of S. mombin against DENV-2. This time, the crushed leaves were macerated with a hydroethanol solution (70:30 v/v) for seven days. They were also submitted to a decoction using distilled water for 15 min. Then, both extracts were lyophilized, resulting in a crystalline powder. To prepare the samples, the powders were diluted in dimethylsulfoxide (DMSO). After the culture of Vero cells was infected, the experiment was carried out for 24 - 168 hr. Then, the action of the extracts was evaluated by quantifying the viral load by real-time PCR (qRT-PCR). No reduction in the viral load was observed, considering all the concentrations tested (0.01 to 100 mg/mL).
The different results found in these two studies,[6,31] are probably due to some differences in the composition of the leaves, even though they are from the same species, and due to differences in other factors or extraction methods.
To evaluate S. mombin’s activity against the HSV-1 virus, Siqueira et al. (2020)[32] carried out an MTT assay to determine cytotoxicity with hydroalcoholic extract, fractions, geraniin – one of the isolated constituents of the extract –, and acyclovir as a control. To perform the antiherpes experiment, the HSV strain was used, as mentioned. For this purpose, an in silico approach was also conducted using a 3-dimensional model of geraniin and the HSV-1 glycoproteins gB and gD as targets.
Considering the cytotoxicity test, fraction C showed the best 50% cytotoxic concentration (CC50) (45.76 ± 0.01 µg/mL) among the other samples when compared to the S. mombin extract (494 ± 85.8 µg/mL) and acyclovir (481.4 ± 112.8 µg/mL). Observing the IC50, although all the extracts showed great results, the best IC50 was observed in geraniin (< 0.09) compared to HE (1.13 × 10−1) and acyclovir (27.39 µg/mL). These results show the important in vitro virucidal activity of geraniin and the tannin fraction. In addition, the in silico approach exhibited a high number of potential strong intermolecular interactions between geraniin and the activity site of the glycoproteins.[32]
Additionally, with the purpose of describing infections caused by the herpesvirus, it is known that herpes simplex viral infection is characterized by an inflammatory process. Because of this, da Silva (2016)[33] decided to study the anti-inflammatory activity of S. mombin, as well as the anti-herpes.
First, the author selected maceration as the best method of extraction. Then, the cytotoxicity was determined by MTT assay using the extract (ESM) and geraniin (GR). The antiherpetic activity of both was verified through the method of Reed and Muench (1938)[34] to obtain the median tissue culture infectious dose (TCID50) and percentage of viral inhibition (% VI).[33]
As a result, no cytotoxicity was observed considering ESM and GR; however, the addition of ESM partially inhibited the PMA-induced [(Phorbol-12-myristate-13-acetate)-induced] release of MPO (myeloperoxidase), with a better effect at the lowest concentration, contrasting with GR, which exhibited an anti-inflammatory activity of 71.8 % at its highest concentration. This could be related to the presence of other secondary bioactive metabolites in the extract. In assessing the antiviral potential, the ESM showed an IC50 of 342.5 µg/mL, GR IC50 of 417.5 µg/mL, and standard IC50 of 570 µg/mL, evidencing antiviral activity.[33]
Antibacterial Activity
Cristofoli et al. (2019)[9] also tested hexane, ethyl acetate, ethanol, water, or a mixture of ethanol:water (1:1 v/v) extracts obtained through different methods, as shown in the Chemical Constituents section. These extracts were diluted in aqueous DMSO solution (60 %), and 50 mg/mL of each was tested against the bacteria Bacillus cereus ATCC 14579, Escherichia coli ATCC 43888, Listeria innocua ATCC 33090, Pseudomonas aeruginosa NCTC 12903, Staphylococcus aureus NCTC 12981, and Saccharomyces cerevisiae NCPF 3178 using the agar diffusion method (ADM).
None of them were able to inhibit P. aeruginosa, and only CO2 + 2.5 % EtOH and CO2 + 2.5 % EtOH + H2O, both obtained through SFE, were capable of inhibiting S. cerevisiae, showing 7.0 and 7.5 mm inhibition zones. When considering the other four strains of bacteria, more extracts proved to be effective: all of them showed good results, presenting inhibition zones between 7.4 and 11.4 mm. The extracts obtained through UAE were shown to be effective against E. coli, achieving similar inhibition zone values between 7.4 and 7.6 mm; the inhibition of 7.6 mm was performed by the aqueous extract. Against S. aureus, the effective extracts were obtained through SFE and Sox, and the best inhibition was 8.0 mm from the extract CO2 + 2.5 % EtOH, obtained through the SFE method. The same extracts also inhibited L. innocua, but CO2 + 5 % EtOH obtained through SFE showed the best result, achieving 11.4 mm. Against B. cereus, the best inhibition zone value was 10.3 mm from the CO2 + 2.5 % EtOH + H2O extract obtained through SFE. It is important to highlight that the results were only compared to the negative control (DMSO), which did not inhibit the microorganisms in the study.[9] If the results were compared to the positive control, the obtained results could be better discussed. Thus, it was possible to observe in this study the importance of performing comparisons among the methods, solvents, and their respective activities, which shows that different results can be obtained from these different parameters.
Another study that demonstrated antibacterial activity was performed by Ajao et al. (1985).[35] After 6 hr of leaf extraction, the researchers tested the aqueous and ethanol (95 %) extracts against Micrococcus luteus, Pseudomonas aeruginosa, Staphylococcus aureus, Escherichia coli, Shigella spp. and Salmonella spp. After evaporation, each extraction residue was dissolved in water. After 24 hr of inoculation, some inhibition was observed at the highest concentration of the extracts (50 % w/w). When the aqueous extract was used against microorganisms, the inhibition zones were between 11 and 21 mm, while the ethanol extract exhibited values between 15 and 21 mm.[35] The negative control with distilled water did not present inhibition; the streptomycin control (10 µg) showed inhibition between 0 and 18 mm, with 18 mm being the best inhibition against M. luteus, which was also the most inhibited by the penicillin control, exhibiting 23 mm inhibition. The inhibition of other strains only achieved values between 0 and 8 mm.
The authors suggested two possibilities for these results: the first is that there is more than one antibacterial component in the extracts, one being more soluble in water and the other being more soluble in ethanol; the second is that there is a single compound soluble in both solvents but much more soluble in ethanol than in water.[35] We observed that these results demonstrate the antimicrobial activity in the S. mombin extract and that they can be useful against S. aureus and E. coli, since the positive controls were not able to inhibit the growth of either microorganism, in addition to its possible action against other strains.
To test the bactericidal activity of S. mombin leaves, Aromolaran and Badejo (2014)[36] conducted tests against four gram-negative isolates: Klebsiella pneumoniae, Serratia marcescens, Salmonella typhi, and Enterobacter aerogenes and one gram-positive isolate: Staphylococcus aureus. After grounding the leaves, extracts with distilled water, ethanol, methanol, and acetone were prepared and tested against the microorganisms.
The aqueous extract exhibited the best results against S. aureus and E. aerogenes, with inhibition zones of 10.67 ± 2.62 and 13.17 ± 1.17 mm; the ethanol extract was the most effective against K. pneumoniae (11.50 ± 1.15 mm) and S. typhi (12.00 ± 1.00 mm); the methanol extract was considered the best against S. marcescens (10.17 ± 0.88 mm), different from the acetone extract, which achieved good values but was not considered the best when compared to other extracts. When using streptomycin as a control, the diameter of inhibition was 12.33 ± 1.09, 08.67 ± 2.98, 11.00 ± 4.31, 12.67 ± 1.42, and 12.33 ± 3.22 mm for K. pneumoniae, S. aureus, S. marcescens, S. typhi, and E. aerogenes, respectively.[36] This study showed the antimicrobial activity of an aqueous S. mombin extract and an organic solvent extract against microorganisms. Olugbuyiro, Moody, and Hamann (2009)[37] conducted an S. mombin cold extraction with methanol. After vacuum liquid chromatography (VLC) using normal phase conditions and hexane, ethyl acetate, methanol, and water as gradient elution, the active portion - named SMi and numbered from 1 to 15 - was submitted to HPLC using water, acetonitrile, and methanol as eluents, which resulted in 22 fractions for the anti-Mycobacterium tuberculosis (antiMtb) test.
The Microplate Alamar Blue Assay (MABA) showed that three fractions resulted in more than 90 % inhibition when a concentration of 64 µg/mL was used: SMi8-9 (94.9 %), SMi14 (98.3 %), and SMi15 (92.8 %), with a focus on SMi15, which was a semipure triterpenoid fraction. Some antibiotics were used as a positive control, obtaining 99.7, 91.4, 99.3, 99.7, and 98.8 % inhibition of rifampin, isoniazid, mox, streptomycin sulfate, and pretomanid, respectively.[37]
When comparing the inhibition obtained through the fractions with that obtained through commonly used antibiotics, it was possible to note that the fractions showed great results against M. tuberculosis, which revealed that S. mombin fractions can be used as probable useful agents in antitubercular drugs.[37]
Leishmanicidal Activity
To verify the leishmanicidal activity, triturated leaves of S. mombin were extracted in methanol:water (4:1) for one week. After isolation and purification, the following fractions were selected to be tested against amastigotes and promastigotes of Leishmania chagasi MHOM46/LC/HZ1: Sm1 - chloroform: ethyl acetate (80: 20), Sm2 – ethyl acetate: chloroform (90: 10), Sm3 – ethyl acetate: methanol (80: 20), and Sm4 – methanol 100 %. These assays were performed by MTT and ELISA methods. A cytotoxicity assay was also performed using RAW 264.7 cells and 100 µg/mL of each fraction and analyzing the percentage of mortality.[38]
The fractions that obtained the best results of leishmanicidal activity were Sm2 and Sm3: Sm2 showed an IC50 of 0.61 µg/mL against amastigotes, while the control (amphotericin B) showed a range of 4.08 – 219.20 µg/mL inhibition. Fraction Sm3 showed the best set of results, exhibiting an IC50 of 0.27 µg/mL against amastigotes and 11.26 µg/mL against promastigotes, in comparison to the control pentamidine, with a range of 0.5 – 52.99 µg/mL, and presented a cytotoxicity of 22.9 % (mortality). The authors suggested that the presence of gallotannins in the extract is probably responsible for leishmanicidal activity in vitro.[38]
After performing ethanol and methanol extractions from the seed and pulp of S. mombin for the phytochemical assay, Asomie et al. (2021)[39] synthesized silver nanoparticles (NPs) using an aqueous extract from the seeds of the plant to evaluate the antibacterial activity. The researchers proceeded with silver nanoparticle (NP) synthesis as follows: 1 g of the pulverized sample was added to 100 mL of distilled water at 60°C for 1 hr, followed by centrifugation. The researchers performed a reaction between the extract (1 mL) and 1 mM AgNO3 (20 mL). Then, the NPs were tested against the following bacterial strains through the agar diffusion method: Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, Klebsiella, Salmonella, Enterobacter, Acinetobacter, Proteus vulgaris, Bacillus subtilis, and Streptococcus pyogenes.
Many different antibiotics were used as controls; for gram-positive strains, Ceftazidime (30 µg), Cefuroxime (30 µg), Gentamicin (10 µg), Ceftriaxone (30 µg), Erythromycin (5 µg), Cloxacin (5 µg), Ofloxacin (5 µg) and Augmentin (30 µg) were selected, while for gram-negative strains, the controls were Septrin (30 µg), Chloramphenicol (30 µg), Sparfloxacin (10 µg), Ciprofloxacin (10 µg), Gentamicin (10 µg), Augmentin (30 µg), Amoxicillin (30 µg), Pefloxacin (30 µg), Tarivid (10 µg) and Streptomycin (30 µg).[39]
With the highest concentration of the extracts (100 µg/mL) in NPs, the inhibition zones were similar to the controls, achieving values of 12 – 15 mm. The results were against E.– 254 and E. coli - ATCC25922 (14 ± 0.1 mm both), E. coli - stool (14 ± 0.2 mm) and Salmonella - 11357 b/d (15 ± 0.2 mm). In some cases, the controls showed better performance against the strains, but the authors considered that NPs with S. mombin extract are a good alternative to the strains’ resistance to commercial and classic antibiotics due to their antibacterial activity evidenced in the study.[39]
Mosquito Adulticidal Activity
To identify mosquito adulticidal activity, Ajaegbu et al. (2016)[40] performed a study using dried and powdered leaves from S. mombin, as described in the Chemical Constituents section. After fractioning the methanol crude extract in different solvents, the samples were dissolved in acetone to obtain concentrations from 1250 to 10000 µg/mL to be tested against Aedes aegypti mosquitoes. To perform this assay, 1 mL of each sample was added to different bottles until all signs of liquid were gone. The mortality of 25 adult female mosquitoes of Ae. aegypti was verified after 24 hr. As a result, the 50% lethal concentration (LC50) values were between 2000 and 5000 µg/250 mL of the extracts, and the best value was 2172.815 µg/250 mL from the dichloromethane fraction. Thus, these experiments show that the S. mombin extracts and their fractions may be utilized for the development of plant-based pesticides.[40]
Anti-inflammatory Activity
A study conducted by Gomes et al. (2020)[8] evaluated the anti-inflammatory activity of the hydroethanol extract (70: 30 - ethanol: water) of S. mombin leaves (HELSM) in an oral mucositis experimental model. Golden Siryan male hamsters were divided into six groups: without mechanical trauma (Normal – N); with mechanical trauma treated with saline solution by gavage (MT); with mechanical trauma treated with saline by gavage and with 5-fluorouracil intraperitoneal injections (5-FU/MT), and the other three groups were treated with HELSM, in which the animals submitted to mechanical trauma received 5-fluorouracil (5-FU) injections and were treated with the S. mombin extract at 50 mg/kg, 100 mg/kg and 200 mg/kg. The results showed that HELSM at a concentration of 200 mg/kg was able to exhibit anti-inflammatory activity in 5-FU-induced oral mucositis in the experimental model, with almost complete healing in all animals, showing re-epithelialization and the absence of hemorrhage, edema, ulcers, and abscesses.[8]
Similar results were also found in the study developed by Cabral et al. (2016).[7] After performing the hydroethanol extraction and obtaining the three major components described in the ‘Chemical Constituents’ section, the authors carried out the experiments to verify anti-inflammatory activity. For this, the researchers selected male BALB/c mice with induced inflammation and pretreated them with saline 0.1 mL/10 g, dexamethasone 0.5 mg/kg, HE at concentrations of 100, 200, 300 or 500 mg/kg, fractions hexane, dichloromethane, ethyl acetate (EtOAc) and butanol at 200 mg/kg (i.p) or chlorogenic acid and ellagic acid (2.5, 5 or 10 mg/kg).
The obtained results demonstrated that HE reduced the amount of leukocyte influx to the peritoneal cavity of treated animals at all concentrations, with emphasis on 300 and 500 mg/kg, which inhibited leukocyte migration by 51.75 and 55.54 %, respectively, indicating decreased inflammation. Considering fractions, EtOAc obtained the best result, with 83.67 % inhibition, which was higher than the other fractions and than HE at all concentrations.[7]
Since ellagic acid and chlorogenic acid were identified in the EtOAc fraction, they were administered in the same biological test at 2.5, 5, and 10 mg/kg, and they presented great results at higher concentrations, such as the inhibition of leukocyte migration of 82 and 70 % induced by ellagic and chlorogenic acids. Thus, this study shows the anti-inflammatory activity of S. mombin leaf extracts and the contribution of both phenolic compounds to this activity.[7]
Antiulcer Activity
Some studies also point out S. mombin with possible antiulcerogenic activity, such as the one developed by Brito et al. (2018).[41] After preparing the extracts and selecting SmEE as the best based on qualitative and quantitative analysis, as mentioned in the Chemical Constituents section, the authors proceeded with the assays to determine the antiulcer activity.
For this, some rats were pretreated orally with 0.9 % NaCl solution (injured control), lansoprazole as the positive control (30 mg/kg), S. mombin ethanol extract (SmEE) (50, 100, and 200 mg/kg), gallic acid (GA, 10 mg/kg), ellagic acid (EA, 7 mg/kg), or gallic acid + ellagic acid (GA + EA, 10 + 7 mg/kg) and were submitted to an ethanol-induced ulcer through the application of absolute ethanol by the oral route. Finally, the animals were euthanized so that it was possible to analyze their stomachs.[41]
As a result, the antiulcer test showed that the administration of SmEE at three concentrations, as well as lansoprazole, was able to inhibit gastric lesions caused by absolute ethanol by 23.84 (50 mg/kg), 90.33 (100 mg/kg), 90.27 (200 mg/kg) and 89.26 % (30 mg/kg), respectively. Oral administration of GA, EA, and GA + EA also reduced gastric lesions by 71.82, 70.92, 94.96, and 92.82 %, showing a synergistic effect when the two substances were used in combination. These results demonstrate the antiulcerogenic activity of the S. mombin ethanol extract and of both gallic acid and ellagic acid compounds, which also presented synergic action to protect the mucosa.[41]
Sabiu et al. (2015)[42] also studied the potential of S. mombin to ameliorate induced gastric ulceration. For this, the researchers extracted 500 g of pulverized leaves with 5 L of distilled water by an orbital shaker. Then, rats were divided in five groups. Group 1 of albino rats received distilled water (normal control), while rats in group 2 received indomethacin; group 3, the S. mombin extract; group 4, indomethacin after pretreatment with esomeprazole; and groups 5 and 6 SM at 100 and 200 mg/kg body weight after ulcer induction with indomethacin. The tests lasted 21 days, and the application of the extracts and the reference drug were performed daily by the oral route. Groups 5 and 6 showed great results of ulcer inhibition, but the best percentage was caused by group 4, showing 83 %.[42] Therefore, this study suggests the ability of S. mombin extract to treat ulcers, as well as the study performed by Brito et al. (2018).[41]
Antidiabetic Activity
The antidiabetic property was also verified in S. mombin by Adediwura and Kio (2009).[43] To perform this experiment, powdered leaves were macerated with 80 % methanol for four days. This extract was suspended in methanol:water (1:9) and partitioned with n-hexane and chloroform, sequentially obtaining the other two fractions. Then, these samples were tested in Wistar albino rats with induced noninsulin-dependent diabetes mellitus (NIDDM). The animals were divided into seven groups: the NIDDM animals in groups 1 - 4 received 1 g/kg methanol extract, n-hexane, chloroform, and aqueous fractions, group 5 received 5 mg/kg glibenclamide (positive control), group 6 received 2 mg/kg water, and a group without NIDDM received 2 mL/kg water (normal rats).[43]
The antidiabetic activity could be evidenced after 7 hr through the oral glucose tolerance test by the methanol extract, which means blood glucose level was 85.3 ± 1.369 (26.3 %) mg/dL, compared to 52.2 ± 0.113 (54.9 %) mg/dL of glibenclamide, 89.0 ± 0.120 mg/dL water (normal rats) and 115.7 ± 0.074 mg/dL of the untreated group. In the alloxan-induced diabetic rats, the methanol extract and the chloroform fraction exhibited a significant decrease in blood glucose levels, with values of 146.6 ± 0.249 (51.1 %) and 186.9 ± 0.014 mg/dL (37.7 %), respectively, which can be compared to the positive control glibenclamide, with 134.6 ± 0.010 mg/dL (55.1 %). The authors suggested that the presence of sterols may be responsible for the antidiabetic activity after observing these results.[43]
Oxytocic Activity
Oxytocic activity was detected in S. mombin leaves. Nworu et al. (2007)[44] prepared a methanol extract (ME) with 500 g of powdered leaves for 72 hr and other extracts such as HF (n-hexane), EF (ethyl acetate), and MF (methanol) with another portion of powdered leaves (500 g). Then, they performed an in vitro assay to detect the uterotonic activity of the samples on gravid and multigravida rat uteri. The obtained results revealed an increased potency of activity in the following order: ME, MF, EF, and HF. For this reason, ME was chosen to further studies.
After this, the acute toxicity/lethality test of ME by the intraperitoneal route (i.p.) route and the estimation of the median effective abortifacient dose (ED50) using mice in the third trimester of pregnancy and administration of ME intraperitoneally resulted in an 50% lethal dose (LD50) of 282.84 mg/kg and an ED50 of 105.53 mg/kg, respectively.[44]
Then, to observe the effect of ME on pregnant rats, the extract was given at three different times: the first group (first trimester) received the extract in the eight first days, while the second group (second trimester) received it between the eighth and fifteenth days, and the third group (third trimester) received it between the sixteenth and twenty-first days of pregnancy.[44]
In each trimester, there were a total of five rats in the study. Of these rats, 80 % suffered abortion in the third trimester, while two suffered intrauterine death in the first trimester, one in the second trimester, and one in the third trimester. It is important to note that two rats in the second and third trimesters died after one week.[44]
Thus, these results show the oxytocic activity of S. mombin leaves. According to the authors, it justifies its use by traditional birth attendants in labor induction, augmentation, and postpartum astringent.[44]
Antifertility Activity
Uchendu and Isek (2008)[45] conducted research using S. mombin leaves to verify the antifertility activity. The powered leaves were cold extracted with petroleum ether and subsequently with 70 % aqueous ethanol. Due to the ability to contract the isolated uterine muscle of rats, the hydroethanol extract was chosen to proceed with the experiments. To test acute toxicity, the rats were treated with 500 mg/kg, 1 g/kg, or 2 g/kg extract intraperitoneally. To determine the anticonceptive and/or abortifacient effect, three groups of pregnant female rats were chosen.[45]
To verify the anticonceptive effect, the first group received 800 mg/kg of the extract dissolved in 70 % aqueous ethanol intraperitoneally for four days, starting for the first day of pregnancy. To verify the abortifacient effect, the same dose was applied to the second group, starting on the eighth day until the eleventh day. The third group was used as a control and did not receive the extract.[45]
The estrogenic activity was also verified as follows: first, ovariectomy was performed, and after fifteen days, the animals were divided into four groups, in which the first was the positive control, by the administration of stilboestrol; the second received the extract at a dose of 500 mg/kg; the third was the negative control, receiving only paraffin oil; and the fourth group was the animals that were neither ovariectomized nor treated.[45] The results showed that only 40 % of the rats treated with the extract were pregnant, showing its antifertility activity. No estrogenic activity was confirmed, since the uterine ratios were 0.985 ± 0.164, 1.718 ± 0.350 (intact groups), and 1.868 ± 0.231 (positive control), and no acute toxicity was verified.[45] Although other experiments, as developed by Nworu et al. (2007),[44] cite abortion as one of the effects of the extract, this phenomenon was not observed in this assay, so the authors suggested that the presence of the increased plasma progesterone levels at the 8th - 11th days may explain the absence of this effect.[45]
Anthelminthic Activity
Aqueous and ethanol extracts were obtained from powdered leaves of S. mombin to verify the anthelminthic activity in in vitro and in vivo tests. First, the extracts were diluted in propylene glycol, achieving concentrations from 0.25 to 2.0 mg/mL. Then, the nutritive medium was added to a suspension containing approximately 100 nematode eggs (with no specific species cited) until they evolved into the first-stage larvae so that 300 µL of the extracts could be added. After seven days, the larvae were in the third stage and counted to verify mortality. The LC50 values of aqueous and ethanol extracts of S. mombin were 0.907 and 0.456 mg/mL, respectively.[46] It was not found the control LC50.
Moreover, in vivo tests were conducted using four groups of lambs (A - D) with natural parasitic infections. Group A was the control, and in B, C, and D, the crude ethanol extract was administered at 125, 250, and 500 mg/kg. Five nematode eggs were identified, and the percentage reduction of fecal egg counts of Hemonchus spp. achieved maxima of 5.6 (group B), 11.0 (group C) and 15.0 % (group D). Regarding Trichostrogylus spp., the values were 9.0, 25.0, and 28.0 % in groups B, C, and D. Esophagostomum spp. and Strongyloides spp. exhibited reductions of 10.1 (group B), 62.0 (group C) and 65.0 % (group D). Trichuris spp. showed a maximum of 15, 100.0, and 100.0 % reductions in groups B, C, and D, respectively. These results demonstrated a dose-dependent effect of the S. mombin extract on these nematode species. According to the authors, this study justifies the popular use of this plant in worm control.[46]
Behavior and Mental Disorders
Sampaio et al. (2018)[47] evaluated the anxiolytic and antidepressant effects of S. mombin leaves on zebrafish (n= 12/group). After obtaining the hydroethanol extract from S. mombin leaves (HELSm) from the leaves and identifying their constituents, as mentioned in the Chemical Constituents section, the anxiolytic activity was evaluated in an aquarium comprising a black side and a white side. For this, the zebrafish were orally administered (2 µL/animal) 100 mg/kg caffeine, 100 mg/kg caffeine + 25 mg/kg buspirone, 100 mg/kg caffeine + 25 mg/kg HELSm and distilled water (control), and then they were analyzed.
Immersion administration was also performed, with each drug diluted in distilled water; thus, the zebrafish could stay in contact with them for 60 min at the following concentrations: caffeine (100 mg/L), buspirone (25 mg/L), and HELSm (25 mg/L) and after submission to a scototaxis test.[47]
For the antidepressant activity, ethanol 1 %, fluoxetine 20 mg/kg and HELSm 25 mg/kg, and the control were orally administered, which simulated a condition of social isolation. After 60 min, the animals were analyzed. In immersion administration, each drug was diluted in distilled water at the following concentrations: 1 % ethanol, 20 mg/L fluoxetine, and HELSm 25 mg/L. The animals were in contact with the drugs for 30 min and were submitted to the novel tank diving test individually.[47]
As a result of the anxiolytic test by immersion and oral administrations, it was verified that the time spent by zebrafish in the white compartment was much superior to the animals who received the control, with approximately 700 s when caffeine and the HELSm were administered and less than 200 s in the control. Latency - time to enter the white compartment - was much lower when caffeine and HELSm were administered (approximately 150 s) than when the control (300 s) was administered orally and by immersion.[47]
According to Maximino et al. (2010),[48] there is a preference of zebrafish for the dark compartment, and an increased number of fishes going to the white side could demonstrate antianxiety behavior, which can be the case for HELSm. In the evaluation of anxiety and depression, the extract produced a similar effect to buspirone and fluoxetine (standard drugs), suggesting that HELSm can be helpful in these situations. The presence of isoquercitrin can participate in these activities, according to the authors.
Ayoka et al. (2006)[49] studied the effects of methanol and ethanol extracts from S. mombin leaves on hexobarbital-induced sleeping time (HIST) and novelty-induced rearing (NIR) behaviors in mice and rats and amphetamine and apomorphine-induced stereotyped (AAIS) and picrotoxin-induced convulsive behavior in rats, as mentioned. To assess NIR, mice were analyzed individually for 30 min after saline or the tested extract injections, and the researchers observed the number of times the mouse was standing on its hind limb with its forelimbs against the wall.
The assessment of HIST was calculated through the time after treatment with hexobarbital and loss of righting reflex (sleep latency) and the time between loss of righting reflex and the regain of right reflex (sleep). AAIS occurred through observing the rats and their classification on the following scale: 0, absence of stereotyped behavior; 1, intermittent sniffing; 2, constant sniffing; 3, constant sniffing with intermittent licking and/or false-biting; 4, constant licking or false-licking; 5, constant licking; 6, constant biting and moving round; 7, constant biting and resisting to a small area in the cage. The anticonvulsant effect was also studied through strychnine and picrotoxin-induced seizures.[49]
As a result, all the extracts produced a dose-dependent decrease in NIR, but ethanol was most potent, showing several rearings of approximately 10 at the highest concentration (100.0 mg/kg), while normal saline exhibited approximately 170 rearing in mice. In rats, almost 30 rats received normal saline, while the extract exhibited no rearing. Considering HIST, only the aqueous extract reduced the latency of sleep at its highest concentration, in contrast to the ethanol extract, which increased the latency time at the dosage of 12.5 mg/kg. The convulsions induced by picrotoxin were significantly reduced when the methanol and ethanol extracts at 50 and 100 mg/kg were used, in which the ethanol reached only 37.5 % of death at the higher concentration, compared to 100 % when no extract was used. None of them were able to help in strychnine-induced convulsions.[49]
The extracts also exhibited a reduction in AAIS behaviors, and ethanol showed the best performance, which also occurred in the other experiment, since this extract increased hexobarbital sleeping-induced time, reduced amphetamine/apomorphine-induced stereotyped behavior, and novelty-induced rearing behavior in mice and rats. Based on these results, the authors suggested that S. mombin leaves can present potential clinical application in the management of psychiatric disorders.[49]
SIDE EFFECT, CONTRAINDICATION, ADVERSE REACTION, AND PRECAUTION
In the study performed by Silva (2015),[50] a sample composed of total solids from S. mombin leaves (HESm), after hydroethanol (70 %) extraction, was used to treat Wistar rats (Rattus novergicus). After the quantitative analysis of its constituents, the acute toxicity was determined through oral application of HESm at 5 g/kg and observation at 30, 60, 120, 180, and 240 min, as well as 14 days after administration.
To verify repeated dose toxicity in the Wistar rats, the animals were treated with doses of 200, 500, and 2000 mg/kg for 30 days, and at the end, their blood was collected. Necropsy was also performed to analyze the external characteristics of some organs. The treatment of pregnant rats was administered at the same doses of the repeated-dose toxicity test in the first six days to observe reproductive toxicity.[50]
No signs of acute toxicity, death, piloerection, diarrhea, or change in locomotor activity were observed, and only a decrease in food consumption occurred in the first week. Considering the hematological profile of male rats, there was a reduction of 6.1 % in the erythrocyte count at 200 mg/kg and 8 and 6.36 % in the values of average corpuscular volume (ACV) and average corpuscular hemoglobin (ACH), respectively, at 2000 mg/kg and an increase of 20.5, 414 % and 44 % in the range values of red cells, basophils, and monocytes, respectively, at the same dose. No difference was found in the levels of glucose, urea, creatinine, aspartate aminotransferase, total cholesterol, triglycerides, alkaline phosphatase, total, direct and indirect bilirubin, lactate dehydrogenase, and total proteins, but a reduction of 38.76 % in alanine aminotransferase levels and an increase of 27.5 % in glucose levels were observed at 200 mg/kg.[50]
Microscopic analysis of the organs revealed a small lymphocytic infiltrate in the liver of rats at concentrations of 500 and 2000 mg/kg and a small increase in the renal capsular space, but no alterations were found in the other organs. No death was found among the pregnant rats, and no toxicity signs were observed; only a reduction in the gain of corporeal mass and an increase in water consumption were observed. Fetuses, ovaries, and placental masses had no change, and no external macroscopic malformations in the fetuses or changes in the number of implantations and resorption were observed. These results show that no oral toxicity was found, but attention should be drawn to possible maternal toxicity during the preimplantation period.[50]
Another study considering the toxicity of S. mombin was conducted, but this time by Luz (2014).[51] The ethanol extract of S. mombin bark was tested at concentrations of 1000, 750, 500, 250, 100, and 20 µg/mL against Artemia salina nauplii. Toxicity assays were also performed through oral administration of the extract at 2000 mg/kg in Swiss mice. As a result, the LC50 values of 482.5 ± 36.1 and 383.2 ± 34.1 µg/mL at 24 and 48 hr, respectively, showed high toxicity in this predictive experiment with Artemia salina; however, contrasting with this result, there was no acute toxicity at the highest dose (2000 mg/kg) in Swiss mice, in which the animals presented few alterations in the behavioral parameters. No changes were observed in the vital organs, such as the heart, kidney, liver, and lung, and the consumption of water and food remained the same. The authors concluded that the absence of a lethal dose can show its safety for popular uses.[51]
However, Asuquo et al. (2012)[52] concluded that although S. mombin is relatively safe, hepatic and renal toxicity can occur with the prolonged use of its leaf extracts. The experiments were conducted with a sample produced through cold extraction with water or ethanol for 72 hr using fresh leaves. The rats received the extracts daily for twenty-eight days and were divided into five groups: A (control), B (250 mg/kg ethanol extract), C (500 mg/kg ethanol extract), D (250 mg/kg aqueous extract), and E (500 mg/kg aqueous extract). Then, blood was drawn from the heart for further analysis. Some organs, such as the liver, heart, lungs, kidneys, stomach, spleen, gonads, and brain, were excised and weighed at the end of the experiments.
As a result, no toxicity and no death were observed in the acute toxicity test, but body weight loss in the treated animals was noted. A significant decrease in food consumption was recorded in the fourth week of treatment in groups C and E, which exhibited values of 42.54 ± 6.22 g and 56.00 ± 7.96 g, respectively, in contrast to the control, which showed approximately 108.65 ± 6.65 g of food. In the same groups, there was an increase in water consumption during the fourth week of extract administration, with water intakes of 234.88 ± 7.12 (group C), 258.55 ± 8.21 (group E), and 258.55 ± 8.21 mL (control). Brain, kidneys, and spleens were significantly different from control in group E with relative mean weight values of 0.64 ± 0.66, 0.42 ± 0.02, and 0.40 ± 0.05, respectively, while the control showed 0.40 ± 0.05, 0.40 ± 0.05, and 0.52 ± 0.04 to the same organs. Group C also showed a difference in the spleen, with a relative mean weight of 0.41 ± 0.01. Through observation of some liver biochemical parameters, it was possible to note that the low density lipoprotein and albumin values were reduced significantly according to the serum biochemical parameters. The hematological indices remained acceptable.[52]
Moussa et al. (2018)[53] tested the toxicity of an aqueous extract of the stem bark of S. mombin in Winstar albino rats. The extract was made through maceration of 50 g of the leaf powder in 1 L of distilled water, followed by filtration, concentration, and drying in an oven. Three oral doses (250, 500, and 1000 mg/kg) and one control (distilled water) were given to the rats for twenty-eight days, and blood samples were collected on days 7, 14, 21, and 28. At the end of the experiment, the liver and kidneys were removed, weighed, and assessed for necrosis, steatosis, congestion, hypertrophy, and calcification.
The authors observed that the administration of the extract at the two highest concentrations caused a decrease in the red blood cell count, and a decrease in the hemoglobin level reflecting anemia and in hematocrits at all dosages was also noted. Different disturbances of erythrocytes, target red blood cells associated with hypochromia, and schizocytes were significantly observed in the rat blood. The hemolysis test resulted in an increase in this parameter according to the time in the tubes containing 12.5 mg/mL S. mombin extract. After 28 days, the anatomohistological study revealed steatosis and apoptosis in the liver and necrosis and calcification in the kidney at 500 and 1000 mg/kg. These results could demonstrate certain toxicity of the extract of S. mombin ,[53] but future studies must be done to determine a safe concentration for use.
FORMULATIONS
Drugs are rarely administered in their pure form, and the most common way is through formulations or medications. Additionally, a good formulation can increase therapeutic efficacy or decrease adverse effects.[54]
Oiseoghaede et al. (2021)[55] formulated an ethanol (70 %) extract from the leaves of S. mombin and Abelmoschus esculentus obtained through maceration for 72 hr. The extract has antioxidant properties. To produce an emulsion, 1 g of methylcellulose and mucilage were triturated and mixed with a solution of S. mombin (75 mg) and distilled water. Then, 40 mL of liquid paraffin and distilled water were added, resulting in a 37.5 % solution prepared through the addition of 200 mg of the extract in 200 mL of water.
The results showed a yield of 2.31 % of A. esculentus and 25.75 % of S. mombin and high stability of the emulsion, with pH = 6.88. The DPPH assay showed that the introduction of mucilage was adequate for use as a good emulsifier, although it decreased the antioxidant properties of the formulation.[55]
In another study, Guedes (2018)[56] used S. mombin pulp as an active ingredient in cosmetics. Body cream was produced as follows: the raw material in Table 3 was heated to 80°C to form phase 1. For phase 2, the corresponding raw materials were heated to the same temperature. Water at 40°C and xantan gum composed Phase 3. Therefore, phase 1 was poured into phase 2 and then cooled to < 40°C. Finally, the other materials were added, including the fragrance solubilized in propylene glycol, and the pH was adjusted with citric acid.
Table 3: Cosmetic formulations developed with the pulp of S. mombin.
| Formulations Developed with the Pulp of S. mombin | ||||||||
|---|---|---|---|---|---|---|---|---|
| Body cream | Face cream | Shampoo formulation | Conditioner formulation | |||||
| Raw material | Approximate formulation percentage | Raw material | Approximate formulation percentage | Raw material | Approximate formulation percentage | Raw material | Approximate formulation percentage | |
| Phase 1 | Ceto-stearyl alcohol | 4.0 - 6.0% | Sodium cetearyl sulfate | 4.0 - 6.0% | Sodium lauryl ether sulfate 27% | 20.0 - 28.0% * | Ceto-stearyl alcohol | 2.0 - 6.0% |
| Ethoxylated ceto-stearyl alcohol | 2.0 - 3.0% | Ethoxylated ceto-stearyl alcohol | 1.0 - 2.0% | Cocamide propyl betaine | 5.0 - 10.0% | Cetyl alcohol | 0.5 - 3.0% | |
| Mineral oil | 0.4 - 0.6% | D-panthenol | 0.1 - 0.3% | Diethanolamide | 1.0 - 2.0% | Behetrimony chloride | 1.0 - 2.5% | |
| Glycerin | 1.0 - 2.0% | Glycerin | 1.0 - 2.0% | Polyquartenium-7 | 1.0 - 2.0% | Shea butter | 0.10 - 0.70% | |
| D-panthenol | 0.1 - 0.3% | - | - | cetrimonium chloride | 0.50 - 1.50% | Mineral oil | Not described | |
| - | - | - | - | - | Propylene glycol | 0,5 – 1,50% (25% of this to phase 1; 1.25% to the pulp and 50% for the fragrance) | Propylene glycol | 1.0 - 2.5 % |
| Phase 2 | Water | 80% of the total water volume * | Water | 80% of the total water volume * | Water | 15% of total water – heated until 80°C | Water – heated until 80°C | enough |
| EDTA | 0.1 - 0.2% | EDTA | 0.1 - 0.2% | Dissodic EDTA | 0.10 - 0.30% | Guar Hydroxypropyltrimonium Chloride | 0.4 - 0.12% | |
| BHT | 0.01 - 0.02% | BHT | 0.01 - 0.02% | BHT | 0.005 - 0.015% | Tapioca | 0.10 - 0.70% | |
| Guar Hydroxypropyltrimonium Chloride | 0.10 - 0.40% | EDTA | 0.10 - 0.25% | |||||
| - | - | - | - | - | - | BHT | 0.007 - 0.012% | |
| Phase 3 | Water | 10% of the total water volume * | Water | 10% of the total water volume * | - | - | Cetyltrimethylammonium chloride | 2.0 - 5.0% |
| Xanthan gum | 0.05 - 0.015% | Xanthan gum | 0.1 - 0.2% *** | Phase 1 | - | Phase 1 | - | |
| - | - | Aluminum starch octenyl succinate | 0.5 - 0.7% *** | Phase 2 | - | Phase 2 | - | |
| Dimethicone 350 | 2.0 - 4.0% | Dimethicone 350 | 2.0 - 4.0% | S. mombin pulp | 0.5 - 3.0% | Ethoxylated lanolin | 0.10 - 0.40% | |
| Isopropyl myristate | 1.0 - 3.0% | Isopropyl myristate | 1.0 - 3.0% | Dye | Enough to | Dimethicone | 1.0 - 1.8% | |
| Methylclothiazolinone | 0.4 - 0.6% | Methylclothiazolinone | 0.4 - 0.6% | Pearlizing | 1.0 - 2.5% | Fragrance | 0.4 - 0.90% | |
| S. mombin pulp | 0.5 - 3.0% | S. mombin pulp | 0.5 - 3.0% | Fragrance | 0.3 - 0.70% | S. mombin pulp | 0.5 - 3.0% | |
| Remaining raw materials added at the end of the formulation | Glycerin | 1.0 - 2.0% | Glycerin | 1.0 - 2.0% | Methylchloroisothiazolinone | 0.10 - 0.70% | ||
| Propylene glycol | 0.4 - 0.6% | Propylene glycol | 0.4 - 0.6% | Ethoxylated lanolin | 0.30 - 0.60% | Citric acid | Until pH 4.5 - 5.0 | |
| Fragrance | solubilized in propylene glycol ** | Fragrance | solubilized in propylene glycol ** | Methylchloroisothiazolinone | 0.20 - 0.60% | - | - | |
| Citric acid | Until pH 5.0 – 6.0 | Citric acid | Until pH 4.5 - 5.0 | Sodium chloride | Enough to | - | - | |
| - | - | - | - | Dimethicone 350 | 0.50 - 1.50% | - | - | |
| - | - | - | - | Citric acid | Until pH 5.5-7.0 | - | - | |
| References | [56] | [56] | [57] | [57] | ||||
After this, a face cream was also formulated. To form phases 1 and 2, the same procedures as the body cream were performed. In phase 3, water at 40°C was used to disperse xanthan gum and aluminum starch octenyl succinate. The final steps were similar to those performed for the body cream, except for the pH, which was adjusted to 4.5 - 5.0. For a sensory analysis, acceptance tests of the volunteers were used, in which appearance, fragrance, spreadability, softness, luminosity, hydration, and firmness were evaluated.[56]
After concluding the formulations, the researchers interviewed some volunteers regarding the possible purchase of body cream. Their answers pointed out that 37 % of the volunteers would certainly buy the products, and 37 % would possibly buy them. Most of the interviewees approved the items, and 79 % of the facial cream volunteers would certainly buy it, showing a difference that may be related to spreadability.[56]
A hydration analysis with Corneometer CM825 showed that the body product containing 3 % S. mombin kept the skin hydration normal and not dry, as it was before the test performance. The formulations also reduced transepidermal water loss, except for the facial product at a concentration of 1.5 %. According to the author, these results show that S. mombin may be a great component of formulations to treat dried skins.[56]
Two other formulations were developed with the pulp of S. mombin, but this time by Ramos and Silva (2018).[57] For the shampoo formulation, the corresponding raw materials were mixed to form Phase 1 (Table 3). With heated water, the other respective raw materials were solubilized at 80°C to form Phase 2. Finally, Phase 3 was formed by joining phases 1 and 2, followed by the addition of the remaining material.
For conditioner development, the raw material in Table 3 was heated to 80°C to form Phase 1. The raw materials related to Phase 2 were mixed in water and heated to the same temperature. After forming Phase 3, cetyltrimethylammonium chloride was added. Then, the mixture was cooled to < 40°C. Finally, the remaining raw material was added.[57]
After the formulations were developed, some analyses were performed with volunteers. Regarding the brightness of the hair strands, the reflectance (GU) was 3.08, 0.78, 1.64 and 1.56 to 0.8 (without pulp), 1.5 (0.5 %), 3.98 (1.5 %), and 1.06 (3.0 %) after washing, showing by comparison among them and the control, that S. mombin is associated with brightness and that higher concentrations may not be beneficial, unlike when at 0.5 and 1.5 %. It is important to note that the shampoo and the conditioner without pulp were the least pleasing, according to the volunteers. Therefore, the pulp of S. mombin can improve shine, softness, and combing of threads, proving to be a good active ingredient.[57]
NANOTECHNOLOGY
In addition to the study developed by Asomie et al. (2021),[39] which shows the antibacterial activity against different bacterial strains caused by synthesized silver nanoparticles (NPs) with the aqueous extract of S. mombin seeds, another study was carried out to produce NPs, but this time, Tijani et al. (2019),[58] performed a green synthesis of this nanoformulation.
The researchers extracted powdered leaves of S. mombin with water by reflux with 400 cm³ of distilled water for 2 hr. After concentration, 10 cm³ of the extract was added to 100 cm³ of a 0.06 M ammonium paratungstate solution, heating them at 120°C for 30 min. Then, 10 % HNO3 was added to correct the pH to 1 - 4, while 0.5 M NH4OH was used to increase it to between 7, 10, and 13. Finally, the solution was separated from the precipitate.[58]
Quantitative analysis showed the presence of 0.9674 mg/mL total flavonoids, 13.0842 mg/mL total phenols, and 3.5022 mg/mL total tannins. To verify the structural and morphological changes of the nanoparticles at different pH values, high-resolution scanning electron microscopy (HRSEM) was performed, showing that their sizes increased at pH 1, 4, 7, and 10 by 13.8, 14.3, 16.7, and 17.6 nm, respectively. To verify the effects of calcination on the size and shape, the nanoparticles were heated to 250°C, 350°C, 450°C, 550°C, and 650°C for 2 hr each. Their shapes changed according to the temperature: at 250°C, the NPs were highly densely agglomerated and small with no definite or irregular shape; at 350°C, their shapes were spherical with larger agglomerates of large particles; at 450°C, they were thick but spherical, and fewer agglomerated particles were formed; at 550°C, they were spherical, more granular in nature and had a lower dense structure; and at 650°C, there were closely packed spherical-shaped WO3 nanoparticles. These results demonstrate how the formation of nanoparticles occurs by green synthesis with S. mombin leaf aqueous extract and tungsten trioxide.[58]
S. mombin was also used to assist ZnO nanoparticle synthesis in solar cells, according to a study performed by Reshma et al. 2021.[59] The first sample was prepared without the species by dissolving 14.875 g of zinc nitrate and 5.625 g of glycine in 100 mL of distilled water at 400 °C for combustion and then at 500°C to obtain pure ZnO. The second was prepared by zinc nitrate (14.875 g) dissolution in distilled water (50 mL) followed by the addition of S. mombin juice pulp extract (30 mL). ZnO NPs were formed after combustion at 375°C but were collected after calcination at 500°C. X-ray diffraction, field emission scanning electron microscopy (FESEM) and high-resolution transmission electron microscopy (HRTEM) analysis showed nonagglomerated, quasi-spherical particles with diameters of 28.13 and 18.32 nm for samples 1 and 2, respectively. The nanoparticles with the S. mombin extract as the photoanode demonstrate conversion with an efficiency of 0.63 % for a dye-sensitized solar cell produced, which is 12.5 % higher than that synthesized through the traditional method. This method was shown to be simple, economically feasible, and eco-friendly.[59] It could be noted that the addition of S. mombin pulp did not increase the particle size but resulted in even smaller nanoparticle sizes, which can be considered a good result.
PATENTS
After the data survey, 67 patent orders and patents of Spondias mombin were found, as shown in Table 4.
Table 4: Patent orders and patents registered in the databases indicated.
| Patents and Orders of Spondias mombin Available in Databases | |||
|---|---|---|---|
| Code | Deposite Date | Title | Database |
| BR 10 2018 076640 6 | 12/20/2018 | Extract, extraction method, and use of Spondias mombin extract as an antimicrobial agent | INPI |
| BR 10 2018 072527 0 | 11/01/2018 | Ready-to-eat hamburger made with natural waste antioxidant | INPI |
| BR 10 2018 072529 7 | 11/01/2018 | Synthesis of activated carbon using the endocarp of Spondias mombin L. | INPI |
| BR 10 2018 067711 0 | 09/04/2018 | Process for obtaining antioxidant extract derived from residues for application in food products intended for human and animal consumption | INPI |
| BR 10 2018 007764 3 | 04/18/2018 | Phytotherapic of cajazeira leaves with antiviral activity | INPI |
| BR 10 2018 003389 1 | 02/21/2018 | Herbal preparations with anti-inflammatory and antiviral activity from a standardized extract of Spondias mombin L. | INPI |
| BR 10 2017 023586 6 | 11/01/2017 | Pharmaceutical method and formulation with gastroprotective activity from nebulized extract of Spondias mombin | INPI |
| BR 10 2013 023217 3 | 08/12/2013 | Extracts, fractions, isolated compounds and pharmaceutical formulation of Spondias mombin applied in the treatment of inflammation | INPI |
| PI 1104660-0 | 08/30/2011 | Herbal medicine with antiviral activity containing ellagitannins | INPI |
| PI 0610227-1 | 05/09/2006 | Attractive for Anastrepha obliqua fruit fly | INPI |
| PI 0506647-6 | 11/25/2005 | The use of chitosan and Spondias mombin in the preparation of herbal medicines | INPI |
| PI 0404307-3 | 08/19/2004 | Herbal medicine with antiviral activity | INPI |
| PI 0314104-7 | 09/11/2003 | Composition for AIDS treatment and associated conditions | INPI |
| PI 9805479-1 | 11/19/1998 | Phytotherapeutic gel of chitin and Spondias mombin with antiviral properties | INPI |
| PI 9603851-9 | 09/12/1996 | Antiviral phytotherapic from the plant species Spondias mombin jacq | INPI |
| WO2009018363A1 | 02/05/2009 | Spondias mombin L. extract and methods of extracting and using such extract | EPO |
| US2002076450A1 | 06/20/2002 | Cosmetic containing plant extracts, particularly with a depigmenting, anti-radical and anti-aging action | EPO |
| RU2500180C1 | 12/10/2013 | Hog plum food product manufacture method | EPO |
| AU2014392682A1 | 11/05/2015 | Methods of reducing signs of skin aging using a combination of extracts | EPO |
| KR101734802B1 | 11/05/2015 | Topical composition including a combination of extracts for reducing signs of skin aging | EPO |
| US2014023731A1 | 03/18/2004 | Composition for treating AIDS and associated conditions | EPO |
| WO2021000037A1 | 07/01/2021 | Method for obtaining bioactive ingredients, use of subcritical-water extraction process, bioactive ingredient, use of bioactive ingredient and cosmetic composition | EPO |
| JP2009167154A | 07/30/2009 | Blood glucose level elevation-inhibiting agent | EPO |
| JP2001122731A | 05/08/2001 | Cosmetic composition containing moisturizing plant extract | EPO |
| ITMI20011837A1 | 03/01/2002 | Extracts from the fungus Guignardia sp., their uses in pharmaceutical compositions, new isolate compound from the extract of the fungus Gignardia sp. and its use in pharmaceutical compositions | EPO |
| AU2011219481A1 | 09/22/2011 | A composition for treating AIDS and associated conditions | EPO |
| EP0768887A1 | 09/12/1996 | Compositions having antiviral properties and preparation process | EPO |
| EP3122941A1 | 10/01/2015 | Reconstituted plant material and its use for packaging, wrapping and food appliances | EPO |
| GB2500662A | 1002/2013 | Modifying the aroma of green coffee beans | EPO |
| WO2009103137A2 | 08/27/2009 | Process for the fermentation of cocoa beans to modify their aromatic profile | EPO |
| US2004166069A1 | 08/26/2004 | Boosting tyrosinase inhibiting activity of skin whitening and sunscreen compositions | EPO |
| WO2016034751A1 | 03/10/2016 | Biocidal products and use thereof for controlling phytopathogens and pest organisms that harm plants | EPO |
| CN103299998A | 09/18/2013 | Application of ginkgolic acid in killing of blue-green algae | EPO |
| US2006127430A1 | 06/15/2006 | Application of ginkgolic acid in killing of blue-green algae | EPO |
| US2014301958A1 | 10/09/2014 | Transoral methods and compositions for wrinkle reduction and cosmetic lip and facial augmentation | EPO |
| US2004146539A1 | 07/29/2004 | Topical nutraceutical compositions with selective body slimming and tone firming antiaging benefits | EPO |
| US2005048008A1 | 03/03/2005 | Antiaging cosmetic delivery systems | EPO |
| US2005271608A1 | 12/08/2005 | Skin whitening compositions based on hydroxyaryl alkyl ketones and their isosteric derivatives | EPO |
| JP2008245625A | 10/16/2008 | Plant culture apparatus and method for aseptic culture of plant clone using the same | EPO |
| US2017156999A1 | 06/08/2017 | Personal care composition in a dissolvable container | EPO |
| WO2010111745A1 | 10/07/2010 | Compositions and methods for increasing vitamin C uptake into cells and methods for retarding skin aging, lightening skin and modulating hair color | EPO |
| WO2010126794A1 | 11/04/2010 | Methods for increasing growth and yield of plants with methionine compounds | EPO |
| US2004208902A1 | 10/21/2004 | Controlled-release nanodiffusion delivery systems for cosmetic and pharmaceutical compositions | EPO |
| US2004161435A1 | 08/19/2004 | Skin firming anti-aging cosmetic mask compositions | EPO |
| US2004241114A1 | 12/02/2004 | Hair care and nail care compositions based on ion-pair delivery system for gender and ethnic selective applications | EPO |
| WO2009135049A1 | 11/05/2009 | Methods and compositions of plant micronutrients | EPO |
| US2008139507A1 | 06/12/2008 | Method of treating skin condition including acne, skin aging, body odor and diaper rash by zinc zeolite clathrates | EPO |
| WO2020242516A1 | 12/03/2020 | Skin treatment composition and preservation system | EPO |
| US2018368427A1 | 12/27/2018 | Skin treatment composition and preservation system | EPO |
| WO2008125433A1 | 10/23/2008 | Plant tissue with an altered content of a flavonoid component | EPO |
| WO2020252461A1 | 12/17/2020 | Natural skin care compositions and methods for treating oxidative stress and restoring skin health | EPO |
| US2004219124A1 | 11/04/2004 | Cosmetic and pharmaceutical masks based on ion-pair delivery system | EPO |
| US2004228884A1 | 11/18/2004 | Ion-pair delivery system for cosmetic and pharmaceutical compositions | EPO |
| US2011098177A1 | 04/28/2011 | Methods and compositions of plant micronutrients | EPO |
Table 4: Patent orders and patents registered in the databases indicated. At column width
| Patents and Orders of Spondias mombin Available in Databases | |||
|---|---|---|---|
| Code | Deposite Date | Title | Database |
| US2009130154A1 | 05/21/2009 | Topical delivery of biological and cosmetic agents by zeolites | EPO |
| DE4221537A1 | 01/05/1994 | Witch hazel dry extract, process for its preparation and its use as a medicinal product | EPO |
| US2006110415A1 | 05/25/2006 | Topical delivery system for cosmetic and pharmaceutical agents | EPO |
| WO2006053415A1 | 05/26/2006 | Plant extract having matrix metalloprotease inhibiting activity and dermatological uses thereof | EPO |
| JP2018512841A | 05/24/2018 | Bacteria engineered for the treatment of diseases that benefit from reduced gastrointestinal inflammation and/or strengthened gastrointestinal mucosal barrier | EPO |
| WO2021055656A1 | 03/25/2021 | Compositions and methods for modifying a plant characteristic without modifying the plant genome | EPO |
| US10273489B2 | 04/30/2019 | Bacteria engineered to treat diseases that benefit from reduced gut inflammation and/or tightened gut mucosal barrier | EPO |
| FR2773487A1 | 07/16/1999 | Use of crude aqueous, hydroalcoholic and acetonic extracts of geraniums, geraniin or related compounds, for the preparation of analgesic drugs of the central nonmorphine type | EPO |
| US2018312851A1 | 11/01/2018 | Bacteria engineered to treat diseases that benefit from reduced gut inflammation and/or tightened gut mucosal barrier | EPO |
| AU2017213646A1 | 08/23/2018 | Bacteria engineered to treat diseases that benefit from reduced gut inflammation and/or tightened gut mucosal barrier | EPO |
| 20200375887 | 12/03/2020 | Compositions and methods for treating skin | USPTO |
| 20110311661 | 12/22/2011 | Plant extracts and dermatological uses thereof | USPTO |
| 20030072820 | 04/17/2003 | Cosmetic containing plant extracts | USPTO |
This table shows 67 patent applications and/or registrations, which demonstrates wide applicability related to the use of S. mombin as a new method of extraction, as an antimicrobial agent, its use in the food industry, green synthesis, phytotherapy uses, pharmaceutical and cosmetic formulations and new types of delivery systems. Thus, there is great potential for the use of S. mombin, with the possibility of developing new methods and developing new products at, for example, the industrial scale.
CONCLUSION
Many different studies on the chemical composition and biological activity of S. mombin have been developed over the years, and through this literature analysis, it was possible to conclude that, related to the chemical composition, different metabolites can be isolated depending on the extraction method, and almost all the extractions resulted in the isolation of the most common phenolic compounds: ellagic acid, chlorogenic acid, and quercetin.
This plant presents some properties that allow it to be used in pesticides. In vitro and in vivo studies confirmed the popular use of S. mombin, showing antioxidant properties and therapeutic activities such as anti-inflammatory, antiulcer, antidiabetic, antiviral, antibacterial, and anthelminthic activities. It was possible to notice its use in cosmetic formulations and nanoformulations. These same activities and formulations were also explored by different researchers, originating patents.
Thus, this review showed that most of the studies use the leaves of S. mombin to study its chemical composition, often using maceration as the extraction method with hydroethanol solvent and that there is a lack of studies about the microscopy of S. mombin barks, which could enrich further studies. Furthermore, there is great potential to transform S. mombin into products, mainly those of therapeutic and cosmetic interest. For this, it is necessary to continue to develop preclinical and clinical studies. We also suggest the performance of scale-up studies, which will also be needed to accomplish this purpose of development.
ACKNOWLEDGEMENT
The authors would like to thank the National Council for Scientific and Technological Development from Brazil (CNPq) and Farmanguinhos for financial support (PROEP-CNPq grant n°. 407856/2017-0). ACBM would like to thank CNPq for the Scientific Initiation Scholarship (PIBIC Program). TRS, MMHA, and JDC are Master’s degree students in Academic Postgraduate Program in Translational Research in Drugs and Medicines, and they thank the Coordination for the Improvement of Higher Education Personnel (CAPES) and Fiocruz for their scholarships.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ABBREVIATIONS
% VI: percentage of viral inhibition; 5-FU: 5-fluoro uracil intraperitoneal injections; AAIS: amphetamine and apomorphine-induced stereotyped; ABTS+: 2,2-azino-bis-(3-ethyl benzothiazoline-6-sulfonic acid); ACV: average corpuscular volume; ACH: average corpuscular hemoglobin; ADM: agar diffusion method; antiMtb: anti-Mycobacterium tuberculosis test; BHT: butylated hydroxytoluene; CC50: 50% cytotoxic concentration; DAD: diode-array detector; DMSO: dimethylsulfoxide; DPPH: 2,2-diphenyl-1-picrylhydrazyl; EA: ellagic acid; ED50: median effective abortifacient dose; EDTA: ethylenediamine tetraacetic acid; EF: ethyl acetate fraction; ELISA: enzyme linked immuno sorbent assay; EO: essential oil; EPO: European Patent Office; ESI-MS: electrospray ionization mass spectrometry; ESM: Spondias mombin extract; EtOAc: ethyl acetate; EtOH: ethanol; FESEM: field emission scanning electron microscopy; GA: gallic acid; GAE: gallic acid equivalent; GC-MS: gas chromatography mass spectrometry; GR: geraniin; GSH: total glutathione level; HE: hydroethanol extract; HELSm: hydroethanol extract from S. mombin leaves; HESm: total solids from S. mombin leaves (HESm); HF: n-hexane fraction; HIST: hexobarbital-induced sleeping time; HPLC: high performance liquid chromatography; HRTEM: high-resolution transmission electron microscopy; IC50: 50% inhibitory concentration; INPI: Instituto Nacional de Propriedade Industrial; i.p.: intraperitoneal route; LC50: 50% lethal concentration; LC-DAD-MS/MS: liquid chromatography tandem mass spectrometry; LD50: 50% lethal dose; MABA: microplate alamar blue assay; ME: methanol extract; MESSM: methanol extract of S. mombin fruit; MF: methanol fraction; MT: mechanical trauma; MTT: 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide; N: normal (without mechanical trauma); NEU: Neu’s reagent; NIDDM: noninsulin-dependent diabetes mellitus; NIR: novelty-induced rearing; NPs: nanoparticles; ODS: octadecyl phase or reversed-phase C18 Column; PMA: phorbol-12-myristate-13-acetate; MPO: myelo peroxidase; qRT-PCR: real-time polymerase chain reaction; SmEE: S. mombin ethanol extract; Smi: S. mombin fractions obtained by VLC from methanol extract; Sox: extracts obtained through the Soxhlet apparatus; SFE: supercritical fluid extraction; TCID50: the median tissue culture infectious dose; TLC: thin layer chromatography; TPC: total phenolic content; UAE: ultrasound-assisted extraction; USPTO: United States Patent and Trademark Office; VLC: vacuum liquid chromatography.
REFERENCES
1. Yellow Mombin MJ [internet]. Miami: fruits of warm climates; c2021 [cited Apr 21 2021]. Available from: https://hort.purdue.edu/newcrop/morton/yellow_mombin_ars.html.
2. Spruce R. Notas de um botânico na Amazônia. Belo Horizonte, Itatiaia; 2006.
3. Ayoka AO, Akomolafe RO, Akinsomisoye OS, Ukponmwan OE. Medicinal and economic value of S. mombin. Afr J Biomed Res. 2008;11:129-36.
4. Cruz GL. Livro verde das plantas medicinais e industriais do Brasil. Belo Horizonte: Oficinas Gráficas de Veloso; 1965.
5. Duke JA, Vasquez R. Amazonian ethnobotanical dictionary. Boca Raton: CRC Press Press; 1994.
6. Silva A, Morais SM, Marques MMM, Lima DM, Santos SCC, Almeida RR, et al. Antiviral activities of extracts and phenolic components of two Spondias species against dengue virus. JVATiTD. 2011;17(4):406-13.
7. Cabral B, Siqueira EMS, Bitencourt MAO, Lima MCJS, Lima AK, Ortmann CF, et al. Phytochemical study and anti-inflammatory and antioxidant potential of Spondias mombin leaves. Rev Bras Farmacognosia. 2016;26(3):304-11. doi: 10.1016/j.bjp.2016.02.002.
8. Gomes MS, Lins RDAU, Langassner SMZ, da Silveira EJD, de Carvalho TG, Lopes MLDS, et al. Anti-inflammatory and antioxidant activity of hydroethanolic extract of Spondias mombin leaf in an oral mucositis experimental model. Arch Oral Biol. 2020;111. PMID 104664.
9. Cristofoli NL, Lima CAR, Vieira MMC, Andrade KS, Ferreira SRS. Antioxidant and antimicrobial potential of cajazeira leaves (Spondias mombin) extracts. Sep Sci Technol. 2019;54(4):580-90. doi: 10.1080/01496395.2018.1508233.
10. Simões EV. Spondias mombin L. In: Amaral ACF, Simões EV, Ferreira JLP. Coletânea científica de plantas de uso medicinal. Rio de Janeiro: Fiocruz; 2004. p. 165-185.
11. Mattieto RA, Matta VM. Cajá (Spondias mombin L.): [Açaí to citrus]. In: Yahia EM. Postharvest biology and technology of tropical and subtropical fruits. Vol. 2. Oxford, Cambridge, Philadelphia, New Delhi: Woodhead Publishing Limited; 2011. p. 330-53.
12. Tropicos.org [internet]. MO: Missouri Botanical Garden; c2021 [cited Jun 24 2021]. Available from: https://www.tropicos.org/name/1300270.
13. Clement CR. 1492 and the loss of Amazonian crop genetic resources. I. The relation between domestication and human population decline. Econ Bot. 1999;53(2):188-202. doi: 10.1007/BF02866498.
14. De Carvalho JEU, Alves RM. Recursos genéticos de espécies do táxon Spondias na Amazônia Oriental. In: Lederman IE, de Lira Júnior JF, da Silva Júnior JF, editors. Spondias no Brasil: umbu, cajá, e espécies afins. Recife: empresa pernambucana de pesquisa agropecuária, IPA/UFRPE; 2008. p. 69-74.
15. Do Sacramento CK, Souza FX. Cajá (Spondias mombin L.). Série frutas nativas, 4. Jabuticabal Funep. 2000.
16. Rios M. Nda S. Pastore Júnior F. Plantas da Amazônia. Vol. 450 espécies de uso geral. Brasília: Universidade de Brasília, Biblioteca Central; 2011.
17. World Agroforestry Centre. Agroforestree database [internet]. c2004 [cited Mar 04 2004]. Available from: https://www.worldagroforestry.org/output/suitability-key-central-american-agroforestry-species-under-future-climates-atlas.
18. Silva-Luz CL, Pirani JR, Pell SK, Mitchell JD. Flora do Brasil. Anacardiaceae [internet]. Rio de Janeiro: Instituto de Pesquisas Jardim Botânico do Rio de Janeiro; c2021 [cited Apr 21 2021]. Available from: http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB4404.
19. Lorenzi H, Matos FJ. As Plantas medicinais no Brasil – nativas e exóticas. 2nd ed. São Paulo: Instituto Plantarum; 2008.
20. Prance GT, Silva MF, de Manaus Á. Manaus: INPA; 1975.
21. Plantas do Nordeste BR, Universitária, UFRN, editors. Especialmente do Ceará. 4th ed. Fortaleza; 1960.
22. Lozano MB. Contribución al estudio de la anatomia floral y de la polinizacion del jobo (Spondias mombin L.). Caldasia. 1986;15:369-80.
23. Da Silva GA, de Brito NJN, dos Santos ECG, López JA, Almeida MG. Gênero Spondias: aspectos botânicos, composição química e potencial farmacológico. J biol Pharm agric Manag. 2014;10(01):27-41.
24. Justiniano MJ, Fredericksen TS. Nash D. Ecología y silvicultura de especies menos conocidas: azucaró Spondias mombin L., Anacardiaceae. 1st. ed. Santa Cruz: Bolfor; 2001.
25. Azevedo DdM, Mendes AMdS, Figueiredo AFd. Característica da germinação e morfologia do endocarpo e plântula de taperebá (Spondias mombin L.) – Anacardiaceae. Rev Bras Frutic. 2004;26(3):534-7. doi: 10.1590/S0100-29452004000300038.
26. Pizarro APB, et al. Avaliação morfoanatômica do caule primário de Spondias mombin L. I Congresso Sul Brasileiro de Plantas Medicinais, Maringá, PR. Vol. 013; 1999a.
27. Pizarro APB, et al. Avaliação morfológica e química das folhas da espécie Spondias mombin LINNAEU. [Anais da Iii reunião da Sociedade latino-americana de fitoquímica e IX Simpósio latino-americano de Farmacobotânica]. Vol. P15. Gramado, RS; 1999b.
28. Mattiero RdA, Lopes AS, Menezes HCd. Caracterização física e físico-química dos frutos da cajazeira (Spondias mombin L.) e de suas polpas obtidas por dois tipos de extrator. Braz J Food Technol. 2010;13(3):156-64. doi: 10.4260/BJFT2010130300021.
29. Abiodun OO, Nnoruka ME, Tijani RO. Phytochemical constituents, antioxidant activity, and toxicity assessment of the seed of Spondias mombin L. (Anacardiaceae). Turk J Pharm Sci. 2020;17(3):343-8. doi: 10.4274/tjps.galenos. 2020.38801, PMID 32636713.
30. Oladimeji AO, Aliyu MB, Ogundajo AL, Babatunde O, Adeniran OI, Balogun OS. Identification and comparison of the volatile constituents of fresh and dried leaves of Spondias mombin found in North-central Nigeria: in vitro evaluation of their cytotoxic and antioxidant activities. Pharm Biol. 2016;54(11):2674-8. doi: 10.1080/13880209.2016.1178308, PMID 27158981.
31. Lima TLC. Avaliação do potencial antiviral da Annona muricata (graviola) e Spondias mombin (cajá) contra o vírus dengue-2 em cultura de células [dissertation]. Natal: Federal University of Rio Grande do Norte; 2015.
32. Siqueira EMdS, Lima TLC, Boff L, Lima SGM, Lourenço EMG, Ferreira ÉG, et al. Antiviral potential of Spondias mombin L. leaves extract against herpes simplex virus type-1 replication using in vitro and in silico approaches. Planta Med. 2020;86(7):505-15. doi: 10.1055/a-1135-9066, PMID 32247285.
33. da Silva TSJ. Desenvolvimento e otimização do extrato padronizado de Spondias mombin (cajazeira): atividades anti-inflamatória e anti-herpes do extrato e da geraniina [dissertation]. Fortaleza: Federal University of Ceará; 2016.
34. Reed LJ, Muench H. A simple method of estimating fifty percent endpoints. Am J Hyg. 1938;27:493-7.
35. Ajao AO, Shonukan O, Femi-onadeko B. Antibacterial effect of aqueous and alcohol extracts of Spondias mombin, and Alchornea cordifolia - two local antimicrobial remedies. Int J Crude Drug Res. 1985;23(2):67-72. doi: 10.3109/13880208509069004.
36. Aromolaran O, Badejo OK. Efficacy of fresh leaf extracts of Spondias mombin against some clinical bacterial isolates from typhoid patients. Asian Pac J Trop Dis. 2014;4(6):442-6. doi: 10.1016/S2222-1808(14)60603-4.
37. Olugbuyiro JAO, Moody JO, Hamann MT. AntiMtb activity of triterpenoid-rich fractions from Spondias mombin L. Afr J Biotechnol. 2009;8(9):1807-9.
38. Accioly MP, Bevilaqua CML, Rondon FCM, De Morais SM, Machado LKA, et al. Leishmanicidal activity in vitro of Musa paradisiaca L. and Spondias mombin L. fractions. Vet Parasitol. 2012;187(1-2):79-84. doi: 10.1016/j.vetpar.2011.12.029, PMID 22521971.
39. Asomie J, Aina A, Owolo O, Olukanni O, Okojie D, Aina F, et al. Biogenic synthesis and characterization of silver nanoparticles from seed extract of Spondias mombin and screening of its antibacterial activity. Int J Nano Dimens. 2021;12(2):175-85.
40. Ajaegbu EE, Danga SPY, Chijoke IU, Okoye FBC. Mosquito adulticidal activity of the leaf extracts of Spondias mombin L. against Aedes aegypti L. and isolation of active principles. J Vector Borne Dis. 2016;53(1):17-22. PMID 27004574.
41. Brito SA, de Almeida CLF, de Santana TI, da Silva Oliveira AR, do Nascimento Figueiredo JCB, et al. Antiulcer Activity and Potential Mechanism of Action of the Leaves of Spondias mombin L Oxid Med Cell Longev. 2018;2018:1731459. doi: 10.1155/2018/1731459. PMID 29854075.
42. Sabiu S, Garuba T, Sunmonu T, Ajani E, Sulyman A, Nurain I, et al. Indomethacin-induced gastric ulceration in rats: protective roles of Spondias mombin and Ficus exasperata. Toxicol Rep. 2015;2:261-7. doi: 10.1016/j.toxrep.2015.01.002, PMID 28962358.
43. Adediwura F, Kio A. Antidiabetic activity of Spondias mombin extract in NIDDM rats. Pharm Biol. 2009;47(3):215-8. doi: 10.1080/13880200802462493.
44. Nworu CS, Akah PA, Okoli CO, Okoye TC. Oxytocic activity of leaf extract of Spondias mombin. Pharm Biol. 2007;45(5):366-71. doi: 10.1080/13880200701214755.
45. Uchendu CN, Isek T. Antifertility activity of aqueous ethanolic leaf extract of Spondias mombin (Anacardiaceae) in rats. Afr Health Sci. 2008;8(3):163-7. PMID 19357744.
46. Ademola IO, Fagbemi BO, Idowu SO. Anthelminthic activity of extracts of Spondias mombin against gastrointestinal nematodes of sheep: studies in vitro and in vivo. Trop Anim Health Pro. 2005;37:223-35.
47. Dos Santos Sampaio TI, de Melo NC, de Freitas Paiva BT, da Silva Aleluia GA, da Silva Neto FLP, da Silva HR, et al. Leaves of Spondias mombin L. a traditional anxiolytic and antidepressant: pharmacological evaluation on zebrafish (Danio rerio). J Ethnopharmacol. 2018;224:563-78. doi: 10.1016/j.jep.2018.05.037, PMID 29852265.
48. Maximino C, De Brito TM. Dias CAGM, Gouveia Jr A, Morato S. Scototaxis as anxiety-like behavior in fish. Nature. 2010;5(2):221-8.
49. Ayoka AO, Akomolafe RO, Iwalewa EO, Akanmu MA, Ukponmwan OE. Sedative, antiepileptic and antipsychotic effects of Spondias mombin L. (Anacardiaceae) in mice and rats. J Ethnopharmacol. 2006;103(2):166-75. doi: 10.1016/j. jep.2005.07.019, PMID 16188408.
50. Silva ELV. Estudo toxicológico não clínico do extrato hidroalcoólico de Spondias mombin L. [dissertation]. Recife: Federal University of Pernambuco; 2015.
51. Luz SMD. Atividade analgésica e toxicidade da Spondias mombin L. Em modelos animais [monography]. Campina Grande: State University of Paraíba; 2014.
52. Asuquo OR, Ekanem TB, Eluwa MA, Oko OO, Ikpi DE. Evaluation of toxicological effects of Spondias mombin in adult male Wistar rats. J Nat Sci Res. 2012;2(7):144-51.
53. Mousssa G, Alassane T, YaoEmile K, Jean-Baptiste ON’g, Mama K, Bernard DY, AngouePaul Y. Toxicity Assessment of an Aqueous Extract of the Stem Bark of Spondias mombin (Anacardiaceae) in Wistar albino rats. IntJCurrMicrobiolAppSci. 2018;7(1):3625-35. doi: 10.20546/ijcmas.2018.702.426.
54. Aulton ME, Taylor K. Aulton’s pharmaceutics: the design and manufacture of medicines. 5th ed. London: Elsevier Health Sciences; 2017.
55. Oiseoghaede JO, Oyawaluja AA, Odukoya OA, Ilomuanya MO, Yinusa OI, Raji OK. Formulation and Antioxidant Activity Evaluation of Spondias mombin - Abelmoschus esculentus Mucilage Emulsion. Bangla Pharma J. 2021;24(1):17-25. doi: 10.3329/bpj.v24i1.51631.
56. Guedes LM. Desenvolvimento, análise cinética e avaliação sensorial em humanos de formulação cosmética contendo polpa de cajá (Spondias mombin L.) [monography]. João Pessoa: Federal University of Paraíba; 2018.
57. Ramos e Silva J. Desenvolvimento e avaliação sensorial de formulação cosmética capilar contendo polpa de cajá (Spondias mombin l.) [monography]. João Pessoa: Federal University of Paraíba; 2018.
58. Tijani JO, Ugochukwu O, Fadipe LA, Bankole MT, Abdulkareem AS, Roos WD. One-step green synthesis of WO3 nanoparticles using Spondias mombin aqueous extract: effect of solution pH and calcination temperature. Appl Phys A. 2019;125:1-12.
59. Reshma HK, Avinash BS, Chaturmukha VS, Ashok RL, Jayanna HS. Hog plum (Spondias mombin) assisted ZnO nanoparticles synthesis: characterization and its impact on the performance of dye-sensitized solar cells. Mater Today Proc. 2021;37(2):434-9. doi: 10.1016/j.matpr.2020.05.424.
60. Le Cointe P. Árvores e plantas úteis (indígenas e aclimadas): nomes vernáculos e nomes vulgares, classificação botânica, habitat, principais aplicações e propriedades. 2nd ed A Amazonia Brasileira, 3. São Paulo: Companhia editora Nacional; 1947.
61. Delgado HS, Sifuentes TC, Herrera JEH, Ruiz JG, Chora EM, Davila MM, Isern FR. Plantas medicinales de la Amazônia peruana utilizadas por los curanderos, chamanes y herbolarios com fines antiinflamatórios. Iquitos: Instituto Peruano de Seguridad Social; 1998.
62. Estrella E. Plantas medicinales amazonicas: realidad y perspectivas. Lima: Texas Commission on the Arts; 1995.
63. Vieira LS. Fitoterapia da Amazônia: manual de plantas medicinais (a farmacia de Deus). 2nd ed. São Paulo: Agronomica Ceres; 1992.
64. Delgado HS, Sifuentes TC. Plantas medicinales del Jardin Botânico IMET-IPSS. Iquitos: Instituto Peruano de Seguridad Social; 1995.
65. Milliken W. Traditional anti-malarial medicine in Roraima, Brazil. Econ Bot. 1997;51(3):212-37. doi: 10.1007/BF02862091.
66. Roig MJT. Plantas medicinales, [Aromáticas e venenosas de Cuba]. Habana: Cultural; 1945.
67. Delgado HS, Herrera JEH, Sifuentes TC, Ruiz JG, Davila MM, Isern FR. Plantas medicinales de la Amazônia peruana utilizadas por curanderos y chamanes com fines anticonceptivos. Iquitos: Instituto Peruano de Seguridad Social; 1997.
68. Correa JEQ, Bernal HYM. Espécies vegetales promisorias de los paises del convenio Andrés Bello. Tomo I. Letra A. PREVECAB. Serie. Cien Tecnol. 1989;11. Bogota: Guadalupe.
69. Furtado LG, Souza RC, Van Den Berg ME. Notas sobre o uso terapêutico de plantas pela população cabocla de Marapanim. Bol. Mus. Para. Emilio Goeldi. 1978;70(1):1-31.
70. Revilla J. Plantas da Amazônia: oportunidades econômicas e sustentáveis. Manaus: INPA; 2001.
71. Luz FJF. Plantas medicinais de uso popular em Boa Vista, Roraima, Brasil. Hortic Bras. 2001;19(1):88-96. doi: 10.1590/S0102-05362001000100019.
72. Amorozo MCM, Gey A. Uso de plantas medicinais por caboclos do Baixo Amazonas. Barcarena, PA, Brasil. Bol. Mus. Para. Emilio Goeldi. 1988;4(1):47-131.
73. Akubue PI, Mittal GC, Aguwa CN. Preliminary pharmacological study of some Nigerian medicinal plants. 1. J Ethnopharmacol. 1983;8(1):53-63. doi: 10.1016/0378-8741(83)90089-2, PMID 6632937.
74. Matta AA. Flora médica brasiliense. 3rd ed. Serie Poranduba, 3. Manaus: Editora Valer; 2003.
75. Barbosa WC, de Nazare RFR, Hashimoto K. Estudo bromatológico e tecnológico da graviola e do tepereba. In: Encontro de Profissionais da química da amazônia. Vol. 1e 2; Anais.. São Luís: CRQ; 1981. p. 307-18.
76. Revilla J. Plantas úteis da Bacia Amazônica. Vol. 2. Manaus: INPA; 2002.
77. Matos FJA. Farmácias vivas: sistema de utilização de plantas medicinais projetado para pequenas comunidades. 3rd ed. Fortaleza: UFC; 1998.
78. Milliken W, Albert B. The use of medicinal plants by the Yanomami Indians of Brazil. Econ Bot. 1996;50(1):10-25. doi: 10.1007/BF02862108.
79. Villachica H. Frutales y hortalizas promissórios de la Amazônia. Lima: Texas Commission on the Arts; 1996.
80. ARde C. A. Cura pelas plantas e diversos meios de grande poder curativo. 3rd ed. São Paulo: Folco Masucci; 1972.
81. Guimarães EF, Rizzini CT, Mautone L, Mattos Filho AM. Árvores do Jardim Botânico do Rio de Janeiro. Rio de Janeiro: Jardim Botânico; 1993.
82. Rodrigues KF, Hesse M, Werner C. Antimicrobial activities of secondary metabolites produced by endophytic fungi from Spondias mombin. J Basic Microbiol. 2000;40(4):261-7. doi: 10.1002/1521-4028(200008)40:4<261:: AID-JOBM261>3.0.CO;2-D, PMID 10986672.
83. Rodrigues KF, Samuels GJ. Fungal endophytes of Spondias mombin leaves in Brazil. J Basic Microbiol. 1999;39:15-8.
84. Corthout J, Pieters L, Claeys M, Geerts St, Vanden Berghe DV, Vlietinck A. Antibacterial and molluscicidal phenolic acids from Spondias mombin. Planta Med. 1994;60(5):460-3. doi: 10.1055/s-2006-959532, PMID 7997478.
85. Sierra MN, Buchelli VF. Reproduction disorders in the mouse caused by Spondias mombin. Rev Colomb Cienc Quim Farm. 1986;15:63-70.
86. Junior S. Plantas exóticas. [Coleção edições do Pasquim]. Vol. 81. Rio de Janeiro: Codecri; 1981.
87. Corthout J, Pieters L, Claeys M, Berghe DV, Vlietinck A. Antiviral caffeoyl esters from Spondias mombin. Phytochemistry. 1992;31(6):1979-81. doi: 10.1016/0031-9422(92)80344-E.
88. Harbourne JB. Phytochemical methods: A guide to modern techniques of plant analysis. 2nd ed. London, New York: Chapman & Hall; 1984.
89. Trease GE, Evans WC. A textbook of pharmacognosy. 13th ed. London: Baulliere Tinall Ltd; 1989.
90. Persinos GJ, Quimby MW. Nigerian plants. 3. Phytochemical screening for alkaloids, saponins, and tannins. J Pharm Sci. 1967;56(11):1512-5. doi: 10.1002/jps.2600561129, PMID 6060599.
91. Wall ME, Eddy CR, McClennan ML, Klumpp ME. Detection and estimation of steroidal sapogenins in plant tissue. Anal Chem. 1952;24(8):1337-41. doi: 10.1021/ac60068a018.
92. Sofowora L. Medicinal plants and traditional medicine in Africa. Ibadan: Spectrum Books Ltd; 2013.
Cite this article: Maria , A. C. Brito, Simões, T. Reis, Ramos, A. de Souza, de Almeida, M. Martins Haddad, da Silva, M. Athana Mpalantinos, da Cruz, et al. Spondias mombin L.: An Updated Monograph, Pharmacog Rev. 2022;16(31):45-61.