|
Pharmacogn Rev. 2022;16(31):7-11 A multifaceted peer reviewed journal in the field of Pharmacognosy and Natural Products www.phcogrev.com | www.phcog.net |
Review Article |
Application of Advance Research Tools in Phytopharmaceutical Adulteration and Authentication: The ‘Omics’ Approach
Muthusamy Kalaivani*, Priyanka Chaudhary, Rajeev Singh Raghuvanshi
Muthusamy Kalaivani*, Priyanka Chaudhary, Rajeev Singh Raghuvanshi
Indian Pharmacopoeia Commission, Ministry of Health and Family Welfare, Government of India, Ghaziabad, Uttar Pradesh, INDIA.
Correspondence
Dr. Muthusamy Kalaivani,
Biologics section, Indian Pharmacopoeia Commission, Ministry of Health and Family Welfare, Government of India, Sector-23, Raj Nagar, Ghaziabad-201 002, Uttar Pradesh, INDIA.
Phone no : 0120-2783400
E-mail: [email protected]
History
• Submission Date: 01-09-2021;
• Review completed: 13-10-2021;
• Accepted Date: 24-11-2021.
DOI : 10.5530/phrev.2022.16.2
Article Available online
http://www.phcogrev.com/v16/i31
Copyright
© 2022 Phcog.Net. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International license.

ABSTRACT
Traditional medicines are the basis of plant derived natural products including extracts, fractions, essential oils and active chemicals. From past decade plant derived products become a primary choice for biological and pharmacological research and contribute for the development of various synthetic and semi-synthetic drugs worldwide. Assurance of the safety, quality and efficacy of phytopharmaceuticals has now become a crucial point throughout the world. The present review is focused about the ‘omics’ approaches towards the identification of any adulteration and maintenance of the quality of phytopharmaceuticals. DNA and RNA based genomics and metabolomics profiling of phytopharmaceuticals have been successfully used for the chemical analysis, characterization and quality control of herbal medicines because of their accuracy and reliability. In this regard, present review discusses the potential usage of various advanced biotechniques like DNA fingerprinting, Next Generation Sequencing, Epigenetic profiling, metabarcoding, chemometrics, Single Nucleotide Polymorphism analysis to authenticate the plant based medicines.
Key words: Phytopharmaceuticals, Genomics, Metabolomics, Chemometrics, Epigenetic profiling.
Cite this article: Kalaivani M, Chaudhary P, Raghuvanshi RS. Application of Advance Research Tools in Phytopharmaceutical Adulteration and Authentication: The ‘Omics’ Approach. Pharmacog Rev. 2022;16(31):7-11.
INTRODUCTION
Plants have been substantial source of medicine since prehistoric times and gaining importance for treatment of various ailments in larger chunk of population. Over the last decade, the herbal medicines have become the choice of treatment in developing and developed countries. Now-a-days these herbal medicines are available not only in pharmacy shops but also in supermarkets and e-marketing cites. According to the World Health Organization (WHO), 80% population of developing countries relies on plant derived medicines for their primary health care requirements due to cultural tradition or lack of alternatives and while in developed countries people use traditional medicines as they believe the natural medicines to be safe in use.[1] WHO’s recent report has shown that various countries are acknowledging the role of traditional and complementary medicines (T&CM) in their national healthcare systems. WHO technical series (WHO-TRS) on Primary Health care on traditional and complementary medicines reported that 124 member states have National laws or regulations for herbal medicines. The safety, efficacy and quality control, have become important matter for both healthcare authorities and the public with the growing use of herbal medicines. Loop holes in regulation and variation in legal laws leads to poor quality and adverse effects of herbal products. In view of this several regulatory authorities have set high requirements in the form of pharmacopoeias and guidelines for the manufacturing and marketing of medicinal plants and related products.
Phytopharmaceuticals are regulated globally under different categorisations in all over the world, as ‘botanicals’ by USA, ‘traditional medicinal product’ by EU, Traditional Chinese Medicines in China, Kampo in Japan, ‘Ayurveda’, ‘Siddha’, ‘Unani’ medicines and ‘Phytopharmaceuticals’ by India.[2] Trade globalisation had expanded the market of these phytopharmaceuticals all over the world. Increasing demand for phytopharmaceuticals is leading to adulteration, which is becoming the big pitfall for health of consumer/patients.[3] Many times adulteration may be due to accidental, misidentification, confusion from vernacular names and lack of authentication.[4,5] Quality, safety and efficacy of plant products are the critical attributes and concern for all regulatory authorities including WHO.[6] World Health Organization and European Medicines Agency (EMA) Issued the guidelines to define the basic criteria for the evaluation of quality, safety, and efficacy of herbal medicines with the goal of assisting national regulatory authorities, scientific organizations, and manufacturers in assessing documentation, submissions, and dossiers in respect of such products. WHO requires data to prove plant drugs to be free from any adulteration and substitution, intra-species variation, contaminations due to pesticide, aflatoxin, heavy metals and microorganisms.[6] Heterogeneity of herbal drug due to intra-species variation and varying in active constituent due to variation in geographical distribution leads to possible uncertainty in its nature and concentration of active constituents which further leads to difficulty in ensuring consistent therapeutic efficacy.[7] Development of suitable standards for phytopharmaceuticals is quite challenging as the preparation can be influenced by a Number of factor including are of origin, age of the plant, yield, cropping time, method of extraction of active moiety and drying, storage condition, process of manufacturing, packaging etc.[8-11] Safety and efficacy of phytopharmaceutical depends directly on the accurate identification and quality assurance (QA) of the starting/raw material. For proper standardization of phytopharmaceutical preparations most of the pharmacopoeias and regulatory guidelines suggest quality attributes like botanical identification, pharmacognostic evaluation, and chemical characterization using various chromatographic methods.[12-14] These existing methods have their own limitations because the composition and relative amount of chemicals in a particular species of plant varies with geographical and environmental conditions, cropping period, storage and processing conditions. However, one of the substantial difficulties associated to quality is that commercial phytopharmaceuticals are either in crushed or in powdered form that consist mixture of two or more herbs in single formula, making it difficult to identify phenotype. Mostly, pharmacopoeial monographs consider single constituents of high relevance in assay and identification methods but in most cases these active constituents are considered as purely analytical markers without any correlation to quality or efficacy of drug.[15-18] In view of these limitations there is a need for error free identification of phytopharmaceuticals with required therapeutic efficacy and safety. However, in the era of phytopharmaceuticals, to understand the cells regulation of gene expression in development and production of phytopharmaceuticals, it is significant to study modification in critical molecular markers at genome and metabolome level which give highly reliable data for informative polymorphisms, as the genetic configuration is distinctive for each species.[19] To understand the diversity of the plant species, techniques using genetic markers are always proved to be an important tool due to its cost effectiveness, high-throughput, convenience and automation.[20-22]
In light of current scenario, present review is focused on current approach for unique identification of phytopharmaceuticals. Earlier, conventional macroscopic, chemical and molecular biology techniques are utilized for plant authentication or adulteration. Nowadays ‘omics’ based approach like genomics and metabolomics authentication is used to identify inter and intra-species variation which is indistinguishable by conventional microscopic, macroscopic, phytochemical and analytical methods.
Genomics approach for identifying adulteration in Herbal Drugs
Nucleic acid (RNA and DNA) is species specific and can be used to identify the phytopharmaceuticals unambiguously, as the species specific unique genetic structure are not affected by age, environmental factors, physiological and storage conditions.[19]
DNA Barcoding
DNA barcoding is a puissant method for the recognition of plant species and its extracts, including phytopharmaceuticals.[4] Method uses short DNA regions of the genome as taxon barcodes for overlaying intra-specific and inter-specific genetic variations in plants and it facilitates fast and correct identification of unidentified herbal samples or formula. DNA barcodes are archived in sequence library of Barcode Life Database (BOLD, www.barcodeoflife.org). Consortium for Barcode of Life Plant working Group assessed seven chloroplast genomic regions across the plant kingdom and, proposed that a combination of matK, iTS2, and rbcL as plant barcodes for maximum species discrimination. However, DNA barcoding in plants does have restrictions, including overlapping intra-specific and inter-specific genetic variations in some groups of plants, limited applicability of chloroplast markers for identification of hybrid species and inability to amplify marker regions due to degraded DNA in processed samples/products.[23] A schematic representation of DNA barcoding is defined in Figure 1.
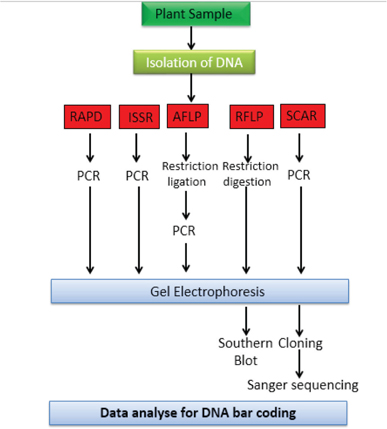
Figure 1: Diagrammatic representation for DNA Barcoding.
Regulatory authority US-FDA and Pharmacopoeias like Indian Pharmacopoeia, United States Pharmacopoeia, and European Pharmacopoeia also recommended DNA barcoding for Botanicals or herbal drug authentication, which reduce the intra-species variation, misidentification and adulteration of herbal drugs or plant material.[7,16-18] Scientific studies have also warranted the use of DNA barcoding for identification of phytopharmaceuticals.[24-27] It’s results are not influenced by cropping period of plants, environmental factors, growth conditions, area of cultivation, drying and storage conditions. This technique is proved to be a boon in case of those plant species which are adulterated frequently with other indistinguishable species or varieties.
Next Generation Sequencing (NGS)
“Next generation sequencing” is one of the quick and cost effective method for entire genome sequencing, it gives information about the species variation, products derived from various species and samples containing fillers or contaminants.[28] The most commonly used NGS techniques are Pyrosequencing, Illumina sequencing, DNA Nano ball sequencing , SOLiD DNA sequencing, and ion-torrent sequencing. This technology has widespread scientific evidence in the authentication and adulteration of phytopharmaceuticals. Recently NGS in combination with Sanger sequencing was successfully used for taxonomic verification of 15 herbal supplements derived from five medicinal plants including Echinacea purpurea, Ginkgo biloba, Hypericum perforatum, Valeriana officinalis and Trigonella foenum-graecum.[28] In Herb spices market, the NGS can also be used for suitable authentication and characterization of samples of spices, herbs, seasoning products, and materials.[29] (Figure 2)
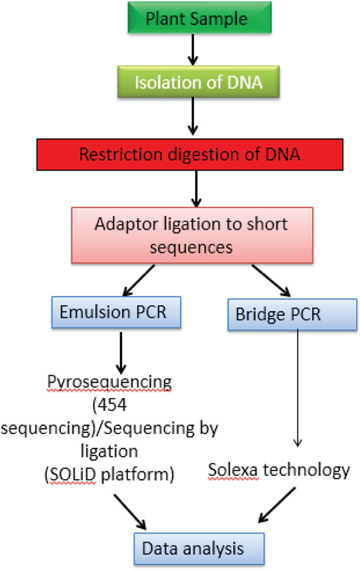
Figure 2: Diagrammatic representation for Next Generation Sequencing technique.
Epigenetic profiling
Epigenetic profiling refers to the study of complex relation between the genome and the environment that are involved in development and differentiation in plants/animals. These changes are inheritable alterations in gene expression without changes in the primary sequence which are already been established and maintained through cell differentiation and division. The major epigenetic mechanism for regulating these inheritable gene alternations are the methylation of cytosine bases in DNA, covalent modifications of histones, post-transcriptional gene regulation by miRNA (microRNA) which can either degrade mRNAs or modulate their translation process.[30] Recently, genetic and epigenetic approaches used for the possible adulteration and auto-adulteration in Saffron (Crocus sativus L.) spice.[31] (Figure 3)
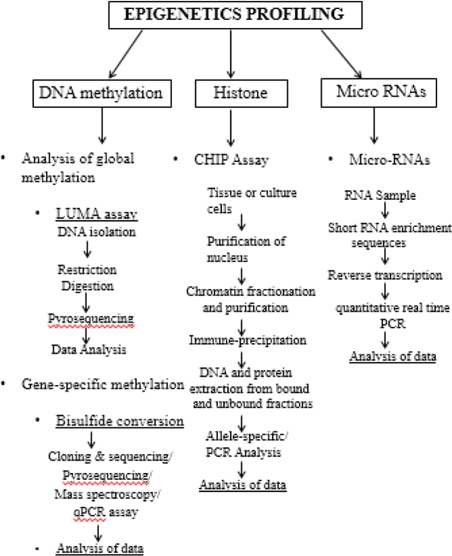
Figure 3: Diagrammatic representation of Epigenetics Profiling technique.
Single nucleotide polymorphism (SNP) Analysis
Single nucleotide polymorphism is a variation in DNA/RNA sequence due to difference in a single nucleotide (A, T, G or C) within same species (Figure 4). SNP are proved to be the most abundant marker system in animal and plant genomes, which is emerging and considered as the molecular markers for identification of heterogeneity and intra-species variation in herbal drugs due to variation in geographical distribution.[32] Gao et al. detected adulterants and substitution in Chinese patent medicines using SNP analysis and only 17 % of the extract and 22% of Chinese medicines were found to be authentic.[33] Scientists are exploring new opportunity to identify novel SNP markers which can be used for variety authentication and functioning regulation at the same time in plants.[34]
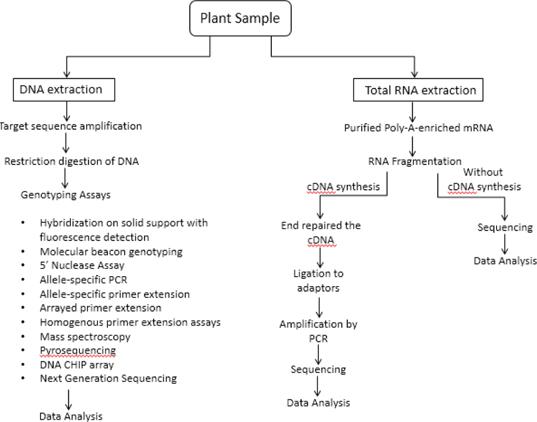
Figure 4: Diagrammatic representation of steps include in Single Nucleotide Polymorphism (SNP) Estimation.
Metabarcoding (Bar-HRM)
Metabarcoding is an advanced technique used for adulteration in herbal products. It is DNA barcoding i.e. combined with High resolution melting, involves monitoring the change in fluorescence occurred through the release of an intercalating DNA dye upon the denaturation of DNA strands. Osathanunkal et al., used Bar-HRM to authenticate three medicinal products of Acanthaceace family in very less time with high accuracy.[35] In another study, Psammosilene tunicoides, an important chinese medicine usually adulterated by related species Silene viscidula. Bar-HRM not only detected the adulteration but also quantified the most Common admixture as low as 1% within relatively very low time.[36,37]
Chemo-metrics
Chemo-metrics is implementation of mathematics and statistics to retrieve more information from the chromatographic data. It provides an ethical opportunity for extracting information regarding chemicals from the original data. Chemo-metrics also offer new ideas and methods for the construction of hyphenated equipment with the advantage of advanced features and computer technology.[38] Recently, chemo-metric analysis of FTIR spectra enabled differentiation between pure passion fruit oil and adulterated samples with unambiguous classification of passion fruit oil products from five different manufacturers.[39] Further, FT-IR and untargeted chemo-metric analysis has also been used for adulterant detection in chia and sesame oils.[40]
Metabolomics approach for herbal drug adulteration
Metabolomics is an ‘omics’ approach concerned with the high-throughput identification and quantification of metabolites present in plants. Profiling of metabolites resolves the metabolic pathways that ultimately represent end products of gene expression. The analytical tools used to analyse the metabolites qualitatively and quantitatively include chromatographic and spectroscopic techniques. Chromatography is used to separate the metabolites which are further quantified and identified and identified with the use of various detectors. Gas chromatography (GC) and high-performance liquid chromatography (HPLC) are two main chromatography systems engaged in metabolic fingerprinting. The detectors used to quantify and identify the metabolites depend largely on the type of components like for a targeted approach, Mass spectroscopy (MS) with chromatography systems like Liquid chromatography-Mass spectroscopy/Mass spectroscopy (LC-MS/MS), Gas chromatography-Mass spectroscopy/Mass spectroscopy (GC-MS/MS) and High Performance Liquid Chromatography-Diode Array Detection (HPLC-DAD) while for untargeted approach Nuclear Magnetic Resonance (NMR), Fourier-transform Infrared Spectroscopy-Attenuated total reflectance (FTIR-AIR), MS and Raman Spectroscopy are used without the chromatography.[41] The steps involved in the process of metabolic fingerprinting are given in Figure 5.
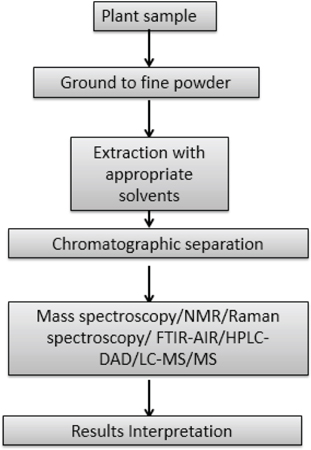
Figure 5: Diagrammatic representation of Metabolic Fingerprinting technique.
Raman spectroscopy is widely used for analysis of chemical compounds in complex mixtures and various authors’ advocates the use of Raman spectroscopy in qualitative and quantitative identification during manufacturing process, chemometric analysis, counterfeit drugs and for screening unapproved drugs.[42-46] Studies also revealed the important role of Raman spectroscopy in quality control of plant based Chinese traditional medicine.[47] Raman spectroscopy is highly reproducible and robust, which can be used without prior separation from multiple components. Hyphenated bio techniques with spectroscopic methods for authentication of herbal medicines warrant the error free identification of phytopharmaceuticals. As discussed earlier, the available traditional techniques can only be used to authenticate the plant material at laboratory level while Raman spectroscopy helps to detect the phytopharmaceuticals effectively and accurately without use of any complicated separation and extraction procedures. Portable Raman spectroscopy could be used to check and identify the raw material, excipients, adulteration and final products from field to market.[47,48]
CONCLUSION
Recently, India had issued a new regulation for new class of plant based drug ‘Phytopharmaceuticals’. Availability, identification and quality of phytopharmaceuticals is the major challenge for the regulatory authorities. The quality may be compromised by adulteration and misidentification of plant materials due to confusion from vernacular names and inter-species variation in various geographical condition.
As the technology advances, plants are being studied bio technically at various levels from DNA to RNA to protein and its complete genome, which provides the opportunity to examine variation within species due to geographical variation and environmental adaptation. Various bio techniques such as DNA Barcoding, Next Generation Sequencing, Epigenetic profiling, meta-barcoding, Single nucleotide polymorphism analysis had been established and studied by various authors for its application in identification of medicinal plants, among which DNA barcoding had been accepted by various regulatory authorities. Similarly many chromatographic and spectroscopic methods had been developed and studied for metabolic profiling of medicinal plants. However, there is no or less effort for combining biotechniques and analytical methods in authentication of medicinal plants.
Scientists have showed promising application of Raman Spectroscopy in Chemical fingerprinting of plants in identifying chemical components such as carotenoids, alkaloids, polyacetylenes, fatty acids, amino acids, terpenoids. They have also used portable Raman spectrophotometer to screen trees using fresh tissues and Raman spectroscopy is capable of detecting chemical differences within the species.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
DECLARATION
The views expressed here are personal and not those of Indian Pharmacopoeia Commission, its scientific committee/groups or any other regulatory agencies.
ABBREVIATIONS
T&CM: Traditional and complementary medicines; EMA: European Medicines Agency; LC-MS/MS: Liquid chromatography-Mass spectroscopy/Mass spectroscopy; GC-MS/MS: Gas chromatography-Mass spectroscopy/Mass spectroscopy; HPLC-DAD: High Performance Liquid Chromatography-Diode Array Detection; NMR: Nuclear Magnetic Resonance; FTIR-AIR: Fourier-transform Infrared Spectroscopy-Attenuated total reflectance.
REFERENCES
1. WHO. WHO guidelines on good agricultural and collection practices (GACP) for medicinal plants. Geneva, Switzerland: World Health Organization; 2003.
2. Sharma S. Current status of herbal product: Regulatory overview. J Pharm Bioallied Sci. 2015;7(4):293-96. doi: 10.4103/0975-7406.168030, PMID 26681886.
3. Seethapathy GS, Tadesse M, Urumarudappa SKJ, Gunaga V S, Vasudeva R, Malterud KE, et al. Authentication of Garcinia fruits and food supplements using DNA barcoding and NMR spectroscopy. Sci Rep. 2018;8(1):10561. doi: 10.1038/s41598-018-28635-z. PMID 30002410.
4. Saslis-Lagoudakis CH, Bruun-Lund S, Iwanycki NE, Seberg O, Petersen G, Jäger AK, et al. Identification of common horsetail [Equisetum arvense L; Equisetaceae] using Thin Layer Chromatography versus DNA barcoding [sci rep:2015. p. 5. p. 11942].
5. De Boer HJ, Ichim MC, Newmaster SG. DNA barcoding and pharmacovigilance of herbal medicines. Drug Saf. 2015;38(7):611-20. doi: 10.1007/s40264-015-0306-8, PMID 26076652.
6. World health Organization (WHO). Quality control methods for herbal materials. Essent Health Prod. 2011;9:167.
7. US Department of Health and Human Services, Food and Drug Administration (FDA). Botanical drug development guidance for industry. Center for Drug Evaluation and Research (CDER) pharmaceutical quality. CMC; 2016.
8. Ernst E. Scientific basis for ayurvedic therapies. Focus Altern Complement Ther. 2004;9(3):243. doi: 10.1111/j.2042-7166.2004.tb04392.x.
9. Van Breemen RB, Fong HH, Farnsworth NR. Ensuring the safety of botanical dietary supplements. Am J Clin Nutr. 2008;87(2):509S-13S. doi: 10.1093/ajcn/87.2.509S, PMID 18258648.
10. Ankli A, Sticher O, Heinrich M. Medical ethnobotany of the Yucatec Maya: Healers’ consensus as a quantitative criterion. Econ Bot. 1999;53(2):144-60. doi: 10.1007/BF02866493.
11. Chen SL, Yu H, Luo HM, Wu Q, Li CF, Steinmetz A. Conservation and sustainable use of medicinal plants: Problems, progress, and prospects. Chin Med. 2016;11:37. doi: 10.1186/s13020-016-0108-7, PMID 27478496.
12. World Health Organization (WHO). Quality control methods for herbal materials. 1998;1:88.
13. Indian herbal pharmacopoeia, Vol. I and II. Jammu: regional Research Laboratory and Mumbai. Indian Drug Manufacturers’ Association; 1998.
14. BHMA. British herbal pharmacopoeia (BHP). Bournemouth: British Herbal Medical Association; 1996.
15. Länger R, Stöger E, Kubelka W, Helliwell K. Quality standards for herbal drugs and herbal drug preparations - Appropriate or improvements necessary? Planta Med. 2018;84(6-7):350-60. doi: 10.1055/s-0043-118534, PMID 28850958.
16. Indian pharmacopoeia. Guidance Manual for Monographs Development of Herbs and Herbal Products including Phytopharmaceutical Drugs. Vol. III; 2016.
17. US Pharmacopeial Convention. USP–NF <41-NF. Vol. 36> Convention. Rockville, MD: U S Pharmacopeial; 2018.
18. European pharmacopoeia (Ph.Eur.). 9th ed. Strasbourg, France: Council of Europe; 2018.
19. Chan K. Some aspects of toxic contaminants in herbal medicines. Chemosphere. 2003;52(9):1361-71. doi: 10.1016/S0045-6535(03)00471-5, PMID 12867165.
20. Rafalski JA, Tingey SV. Genetic diagnostics in plant breeding: RAPDs, microsatellites and machines. Trends Genet. 1993;9(8):275-80. doi: 10.1016/0168-9525(93)90013-8, PMID 8104363.
21. Rafalski JA. Novel genetic mapping tools in plants: SNPs and LD-based approaches. Plant Sci. 2002;162(3):329-33. doi: 10.1016/S0168-9452(01)00587-8.
22. Chen X, Gonçalves MAFV. DNA, RNA, and Protein Tools for editing the genetic information in human cells. iScience. 2018;6:247-63. doi: 10.1016/j. isci.2018.08.001, PMID 30240615.
23. Osathanunkul M, Suwannapoom C, Ounjai S, Rora JA, Madesis P, De Boer H. Refining DNA Barcoding coupled high resolution melting for discrimination of 12 closely related croton species. PLOS ONE. 2015;10(9):e0138888. doi: 10.1371/journal.pone.0138888, PMID 26406615.
24. Ema EMA, CVMP/814/00 Rev. CPMP/QWP/2819/00. Rev EMA/HMPC. 2005;2; 2011:2. PMID 201116. Rev. Guideline on quality of herbal medicinal products/traditional herbal medicinal products.
25. Wallace LJ, Boilard SMAL, Eagle SHC, Spall JL, Shokralla S, Hajibabaei M. DNA barcodes for everyday life: Routine authentication of natural health Products. Food Res Int. 2012;49(1):446-52. doi: 10.1016/j.foodres.2012.07.048.
26. Hall SD, Wang Z, Huang SM, Hamman MA, Vasavada N, Adigun AQ, et al. The interaction between St John’s wort and an oral contraceptive. Clin Pharmacol Ther. 2003;74(6):525-35. doi: 10.1016/j.clpt.2003.08.009, PMID 14663455.
27. Li W, Godzik A. Cd-hit: A fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics. 2006;22(13):1658-59. doi: 10.1093/bioinformatics/btl158, PMID 16731699.
28. Ivanova NV, Kuzmina ML, Braukmann TWA, Borisenko AV, Zakharov EV. Correction: Authentication of herbal supplements using Next-Generation Sequencing. PLOS ONE. 2016;11(12):e0168628. doi: 10.1371/journal. pone.0168628, PMID 27959957.
29. Barbosa C, Nogueira S, Gadanho M, Chaves S. Study on commercial spice and herb products using next-generation sequencing (NGS). J AOAC Int. 2019;102(2):369-75. doi: 10.5740/jaoacint.18-0407, PMID 30609949.
30. Harada BT, He C. Epigenetics: Making your mark on DNA. Nat Chem. 2017;9(11):1040-42. doi: 10.1038/nchem.2884, PMID 29064503.
31. Soffritti G, Busconi M, Sánchez RA, Thiercelin JM, Polissiou M, Roldán M, et al. Genetic and epigenetic approaches for the possible detection of adulteration and auto-adulteration in saffron (Crocus sativus L.) spice. Molecules. 2016;21(3):343. doi: 10.3390/molecules21030343, PMID 26978342.
32. Ibrahim MA, Mäenpää M, Hassinen V, Kontunen-Soppela S, Malec L, Rousi M, et al. Elevation of night-time temperature increases terpenoid emissions from Betula pendula and Populus tremula. J Exp Bot. 2010;61(6):1583-95. doi: 10.1093/jxb/erq034, PMID 20181662.
33. Gao Z, Liu Y, Wang X. Derivative technology of DNA bar coding (nucleotide signature and SNP double peak methods) detects adulterants and substitution in Chinese Patent Medicines [sci rep. p. 2017. p. 7. p. 5858].
34. Wu WR, Cheng CS, Cheng QQ, Lao CC, Cui H, Tang ZY, et al. Novel SNP markers on ginsenosides biosynthesis functional gene for authentication of ginseng herbs and commercial products. Chin J Nat Med. 2020;18(10):770-78. doi: 10.1016/S1875-5364(20)60017-6, PMID 33039056.
35. Osathanunkul M, Madesis P, De Boer H. Bar-HRM for authentication of plant-based medicines: Evaluation of three medicinal products derived from Acanthaceae species. PLOS ONE. 2015;10(5):e0128476. doi: 10.1371/journal. pone.0128476, PMID 26011474.
36. Buddhachat K, Osathanunkul M, Madesis P, Chomdej S, Ongchai S. Authenticity analyses of Phyllanthus amarus using barcoding coupled with HRM analysis to control its quality for medicinal plant product. Gene. 2015;573(1):84-90. doi: 10.1016/j.gene.2015.07.046, PMID 26188160.
37. Li J, Song M, Xiong C, Zhao B, Sun W. Application of barcode high-resolution melting for rapid authentication of the medicinal plant Psammosilene tunicoides. Biotechnol Biotechnol Equip. 2016;30(4):790-96. doi: 10.1080/13102818.2016.1181988.
38. Bansal A, Chhabra V, Rawal RK, Sharma S. Chemometrics: A new scenario in herbal drug standardization. J Pharm Anal. 2014;4(4):223-33. doi: 10.1016/j. jpha.2013.12.001, PMID 29403886.
39. Kiefer J, Lampe AI, Nicoli SF, Lucarini M, Durazzo A. Identification of passion fruit oil adulteration by chemometric analysis of FTIR spectra. Molecules. 2019;24(18):3219. doi: 10.3390/molecules24183219, PMID 31487942.
40. Rodríguez SD, Gagneten M, Farroni AE, Percibaldi NM, Buera MP. FT-IR and untargeted chemometric analysis for adulterant detection in chia and sesame oils. Food Control. 2019;105:78-85. doi: 10.1016/j.foodcont.2019.05.025.
41. Van der Kooy F, Maltese F, Choi YH, Kim HK, Verpoorte R. Quality control of herbal material and phytopharmaceuticals with MS and NMR based metabolic fingerprinting. Planta Med. 2009;75(7):763-75. doi: 10.1055/s-0029-1185450, PMID 19288400.
42. Lawson LS, Rodriguez JD. Raman barcode for counterfeit drug product detection. Anal Chem. 2016;88(9):4706-13. doi: 10.1021/acs.analchem.5b04636, PMID 27043140.
43. Navin CV, Tondepu C, Toth R, Lawson LS, Rodriguez JD. Quantitative determinations using portable Raman spectroscopy. J Pharm Biomed Anal. 2017;136:156-61. doi: 10.1016/j.jpba.2016.12.020, PMID 28081502.
44. Shah RB, Tawakkul MA, Khan MA. Process analytical technology: Chemometric analysis of Raman and near infra-red spectroscopic data for predicting physical properties of extended release matrix tablets. J Pharm Sci. 2007;96(5):1356-65. doi: 10.1002/jps.20931, PMID 17455359.
45. Gowen AA, O’Donnell CP, Cullen PJ, Bell SE. Recent applications of Chemical Imaging to pharmaceutical process monitoring and quality control. Eur J Pharm Biopharm. 2008;69(1):10-22. doi: 10.1016/j.ejpb.2007.10.013, PMID 18164926.
46. Tondepu C, Toth R, Navin CV, Lawson LS, Rodriguez JD. Screening of unapproved drugs using portable Raman spectroscopy. Anal Chim Acta. 2017;973:75-81. doi: 10.1016/j.aca.2017.04.016, PMID 28502430.
47. Chen DD, Xie XF, Ao H, Liu JL, Peng C. Raman spectroscopy in quality control of Chinese herbal medicine. J Chin Med Assoc. 2017;80(5):288-96. doi: 10.1016/j. jcma.2016.11.009, PMID 28325576.
48. Huang CC. Applications of Raman spectroscopy in herbal medicine. Appl Spectrosc Rev. 2016;51(1):1-11. doi: 10.1080/05704928.2015.1092154.
Cite this article: Kalaivani M, Chaudhary P, Raghuvanshi RS. Application of Advance Research Tools in Phytopharmaceutical Adulteration and Authentication: The ‘Omics’ Approach. Pharmacog Rev. 2022;16(31):7-11.